Functional protein and cyanine dye molecule compound and preparation method and application thereof
A cyanine dye, protein technology, applied in material excitation analysis, preparations for in vivo experiments, material analysis by optical means, etc., can solve the problems of easy diffusion in vivo, poor in vivo stability, short imaging window, etc. Signal-to-noise ratio and penetration depth, slower dispersion, and less background scattering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
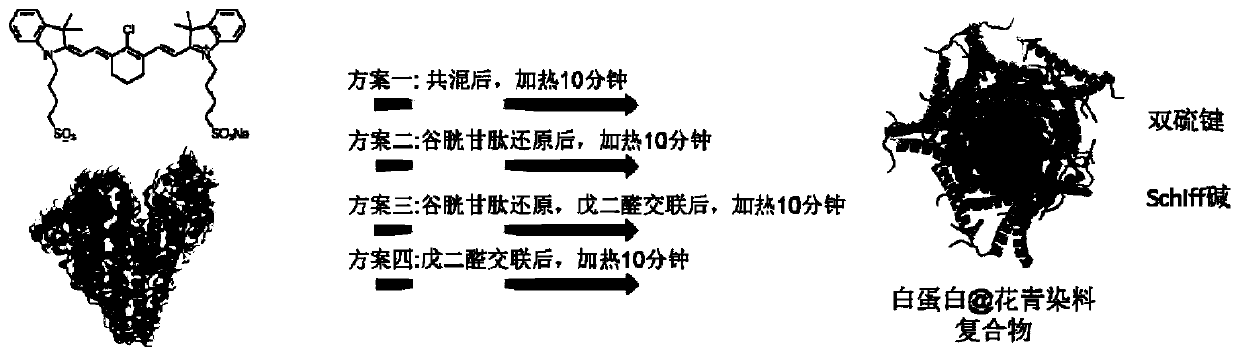
[0058] Select three typical cyanine dyes ICG, IR-783 and IR-12N3 respectively figure 1 The 4 schemes provided are coated with albumin, and the specific schemes are as follows:
[0059] Scheme 1: Add 500 microliters of PBS to 500 microliters of albumin solution, add 11 microliters of cyanine dye, mix well, react in a shaking box at 37 degrees Celsius for 24 hours, wash five times with a 30k centrifugal filter membrane, and post-process The temperature is 60 degrees Celsius for 10 minutes.
[0060] Scheme 2: Add 500 microliters of PBS to 500 microliters of albumin solution, add 60 microliters of glutathione solution and mix well; add 11 microliters of cyanine dye and mix well, react in a shaking box at 37 degrees Celsius for 24 hours, use 30k The centrifugal filter membrane was washed five times, and the post-treatment temperature was 60 degrees Celsius for 10 minutes.
[0061] Scheme 3: Add 500 microliters of PBS to 500 microliters of albumin solution, add 60 microliters of ...
Embodiment 2
[0065] Example 2. Preparation of complexes of IR-783 and albumin by simple blending
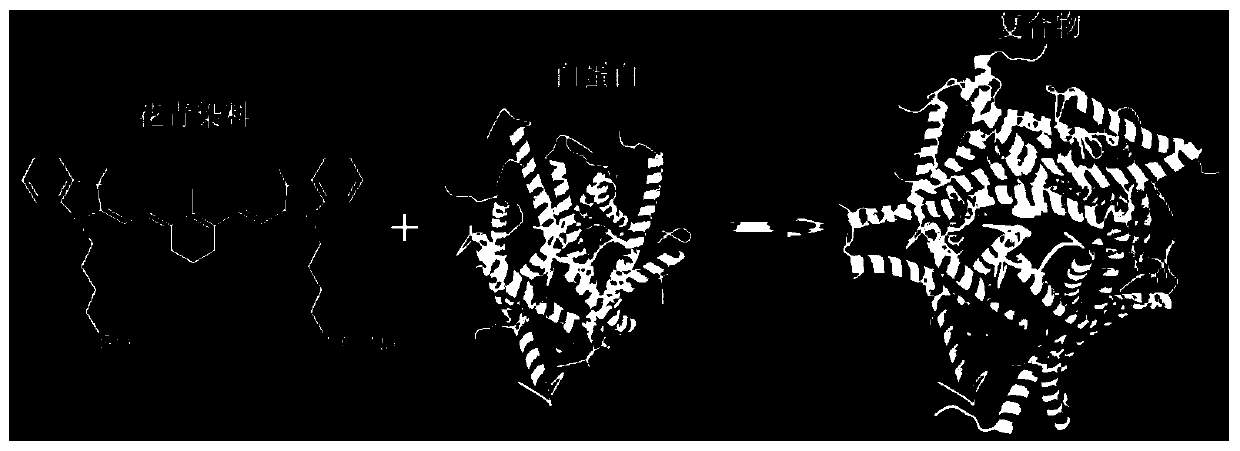
[0066] Preparation scheme such as figure 1 As shown in "Scheme 1", it specifically includes: adding IR-783 solution to 40mg / ml (602μM) BSA solution, controlling the molar ratio of albumin and IR-783 to 1:1, mixing, and controlling albumin The reaction concentration is 20 mg / ml, reacted in a shaking box at 37 degrees Celsius for 24 hours, washed five times with a 30k centrifugal filter membrane, and the post-treatment temperature was 60 degrees Celsius for 10 minutes. The reaction process and the obtained complex structure can be found in figure 1 , 2 Schematic of , nanoparticle-like under the transmission electron microscope, see Figure 8 Shown in the leftmost figure. The resulting complex was designated as "IR-783@BSA".
Embodiment 3
[0067] Example 3. Preparation of complexes of IR-783 and albumin by reduction of glutathione after blending
[0068] Preparation scheme such as figure 1 As shown in "Scheme 2", it specifically includes: adding 60 microliters of 0.25M glutathione solution containing 15% DMSO to 40mg / ml (602μM) BSA solution, and then adding IR-783 solution to control the interaction between albumin and IR-783 reaches a molar feed ratio of 1:1, mixes well; and controls the albumin reaction concentration at 20mg / ml, reacts in a shaking box at 37 degrees Celsius for 24 hours, washes five times with a 30k centrifugal filter membrane, and the post-treatment temperature is 60 degrees Celsius for 10 minutes, the reaction process and the structure of the resulting complex can be found in figure 1 , 2 Schematic of , nanoparticle-like under the transmission electron microscope, see Figure 8 Shown in the second picture from the left. The resulting complex was designated as "IR-783@BSA-GSH".
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com