Application of thyroid beta-agonist
A dose, adrenal brain technology, applied in the field of treatment of X-linked adrenoleukodystrophy, can solve the problem of limited treatment options for X-ALD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0253] Example 1 - Evaluation of different treatment doses
[0254] Two different X-ALD fibroblast cell lines were incubated in duplicate with the four compounds at the three doses as indicated in Table 3.
[0255] table 3
[0256] compound Low middle high 2 100nM 1μM 10μM 4 100nM 1μM 10μM 1 1μM 10μM 100μM 3 1μM 10μM 100μM
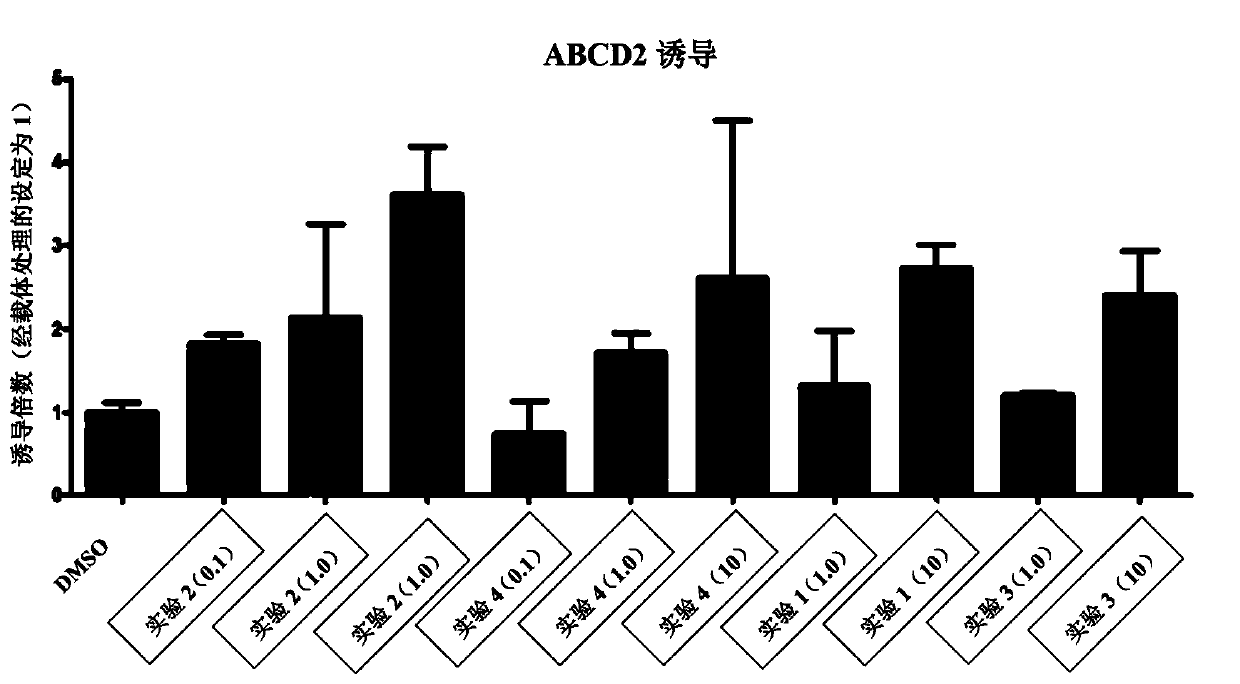
[0257] Two X-ALD cell lines incubated with 100 μM of 1 and 3 died within 24 hours. This strongly suggests that 1 and 3 are toxic at 100 μM. After 72 hours, cells that had received 0.1 μM, 1 μM or 10 μM of 2 or 4, or 0.1 μM or 10 μM of 1 or 3 appeared healthy as judged by their proliferation and morphology.
[0258] After 72h, cells were harvested and mRNA was isolated for QPCR analysis. Such as figure 1 The effect of compounds on ABCD2 expression was compared to ABCD2 expression levels in untreated (DMSO) cell lines as shown in . For all 4 compounds tested, 10 μM was the most effective. A...
Embodiment 2
[0259] Example 2 - Evaluation after prolonged culture
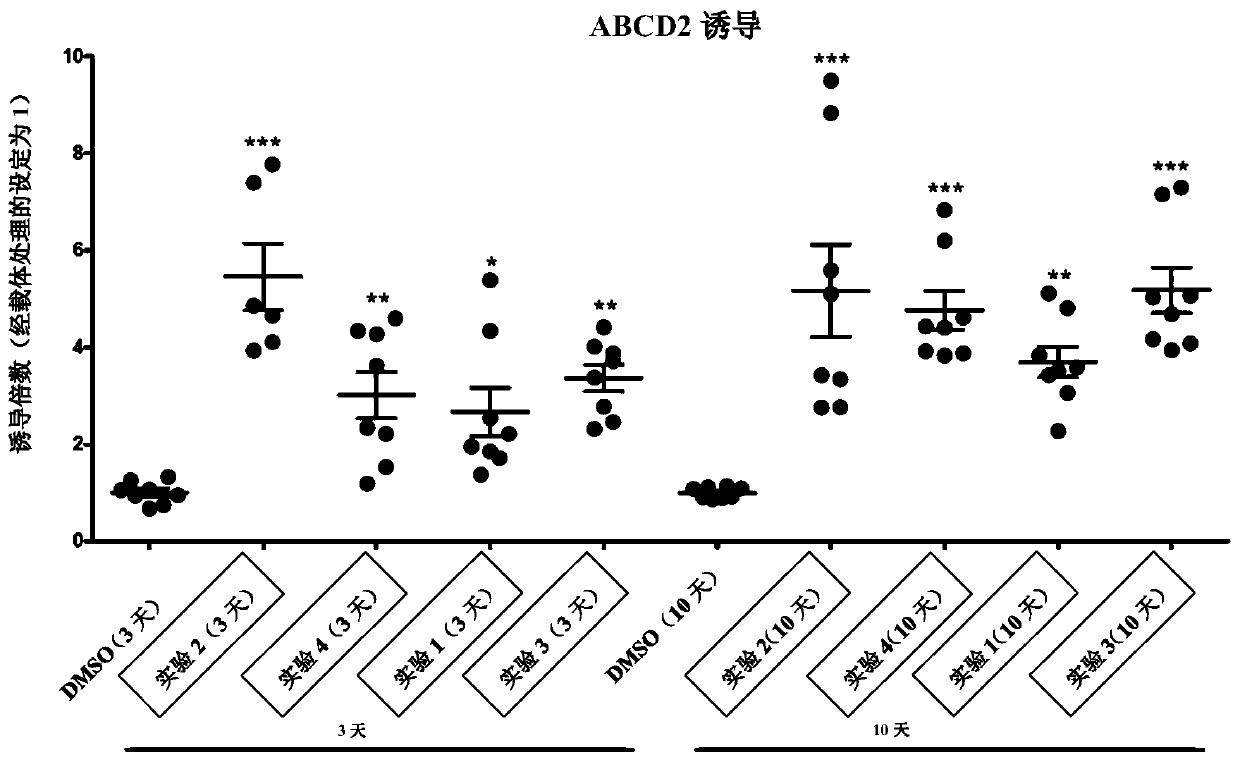
[0260] Four X-ALD cell lines were incubated with four Compound 1, Compound 2, Compound 3 and Compound 4 at 10 μM. The effect of treatment on ABCD2 expression was analyzed at day 3 and day 10. For the 10-day incubation, refresh tissue culture medium and compounds on days 3 and 6.
[0261] Days 3, 6, and 10: All cells appeared healthy, proliferation was normal, and morphology was normal. No abnormality noted. After 3 days, cells were harvested and mRNA was isolated for QPCR analysis. After 10 days, cells were harvested and mRNA was isolated for QPCR analysis. For all samples, cDNA synthesis and QPCR were done on the same day.
[0262] Such as figure 2 The effect of compound treatment on ABCD2 expression was compared to ABCD2 expression levels in untreated (DMSO) cell lines at days 3 and 10, as shown in . Prolonged exposure resulted in comparable effects on ABCD2 induction.
Embodiment 3
[0263] Example 3 - 10-day treatment of VLCFA de novo synthesis
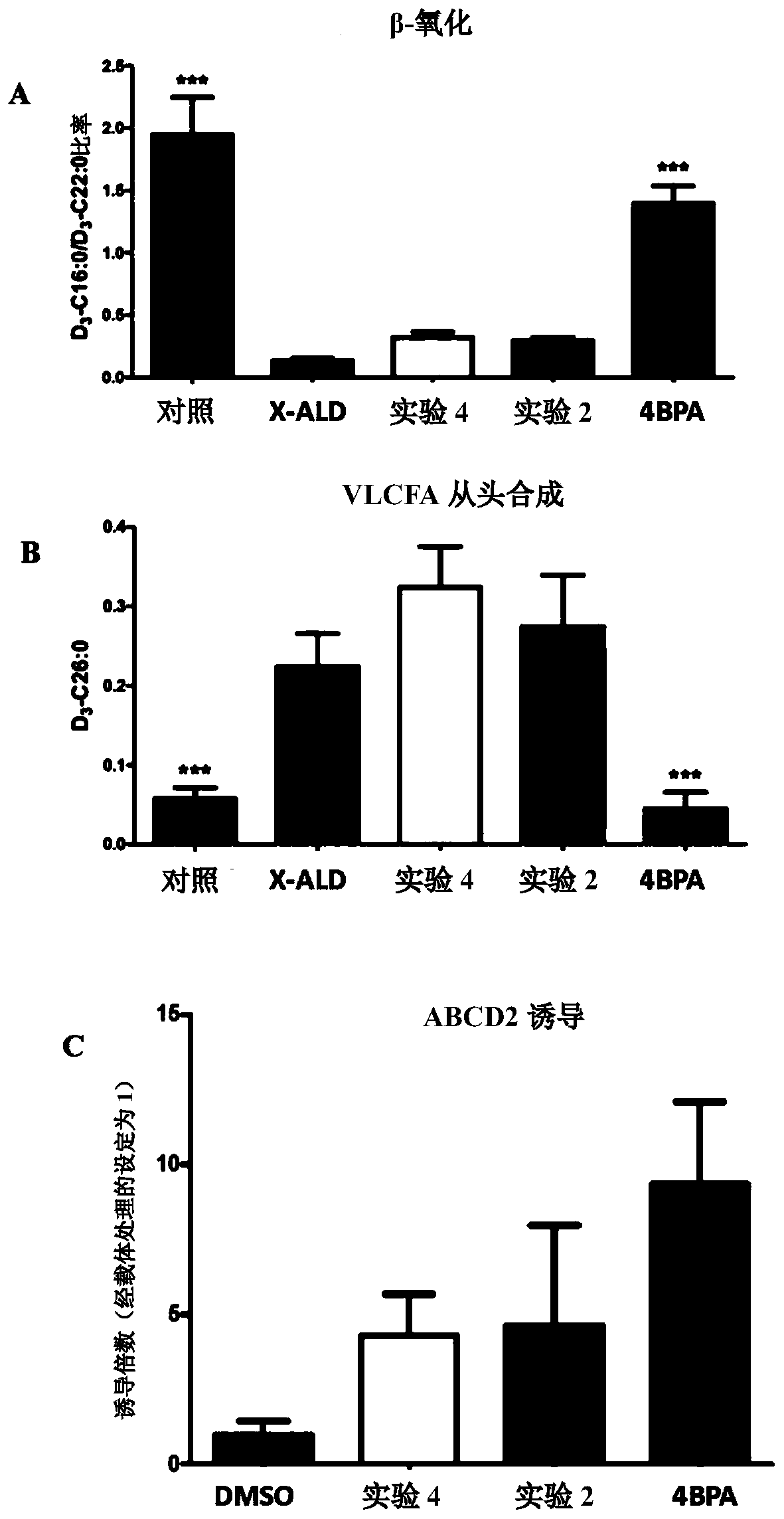
[0264]Five different X-ALD cell lines were incubated with 10 μM of compound 1, compound 2, compound 3 and compound 4, 5 mM 4PBA (sodium 4-phenylbutyrate) or 0.1 μM sobetirome for 6 days. On day 6, 30 μM D3C22:0 was added to assess the effect of treatment on β-oxidation and D3C26:0 de novo synthesis. 6 untreated control cells were included as well as 5 different untreated X-ALD cells to allow assessment of treatment effects. The total consisted of 41 experiments.
[0265] Such as Figure 4 As shown in , in untreated X-ALD cells, β-oxidation capacity was reduced by ~80%, and de novo synthesis of C26:0 was increased by ~4-fold. The positive control (4PBA) restored β-oxidation to -50% of control and normalized VLCFA synthesis to near normal levels. The positive control (sobetirome) did not show any beneficial effect on VLCFA β-oxidation or de novo synthesis.
[0266] Compound 1 (which was also the most active ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com