Preparation method of (R)-chloromandelic acid
A technology of o-chloromandelic acid and reaction buffer, which is applied in the direction of fermentation, can solve environmental hazards, waste liquid discharge and other problems, and achieve the effects of reducing emissions, reducing production costs and improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Specifically, the preparation method of the embodiment of the present invention at least includes the following steps:
[0035] (1) cultivating Escherichia coli engineering bacteria expressing nitrilase;
[0036] (2) adopting the engineered bacterium that cultivates to obtain as catalyst, o-chloromandelonitrile as substrate, and reaction buffer as reaction medium to react;
[0037] (3) After the reaction finishes, separate and collect the supernatant;
[0038] (4) After extracting (R)-o-chloromandelic acid in the supernatant, a flow-through is obtained, and the flow-through is recycled at least once in step (2) as the reaction buffer.
[0039] Specifically, the engineered Escherichia coli bacteria expressing nitrilase used in step (1) can be commercially available strains, and preferably engineered bacteria constructed in the method disclosed in Application No. 201510978973.7.
[0040] Optionally, the culturing in step (1) includes culturing in shake flasks or ferment...
Embodiment 1
[0067] 1. Add 100ml of phosphate buffer (NaH 2 PO 4 / Na 2 HPO 4 Buffer), pH 7.5~8.5, add 1.5g of engineered bacteria cells, 30mM substrate o-chloromandelonitrile and cosolvent (methanol, the amount added is 1% of the volume of the reaction system), react in a shaker at 220rpm, The substrate was fed in batches for 6 times, and the substrate concentration was 25 mM each time, and the end point of the reaction was monitored by thin-layer chromatography. After the substrate has reacted, the insolubles are removed by centrifugation, and the supernatant is transferred to an activated weak base anion resin, the product (R)-o-chloromandelic acid in the adsorption supernatant is collected, and the flow-through is recorded as Reaction solution 1; then use 50ml 2M HCl to elute the product on the resin, collect the eluent, and record it as eluent 1.
[0068] 2. Transfer reaction solution 1 to a 250ml Erlenmeyer flask, add 1.5g of engineering bacteria cells, 30mM substrate o-chloromand...
Embodiment 2
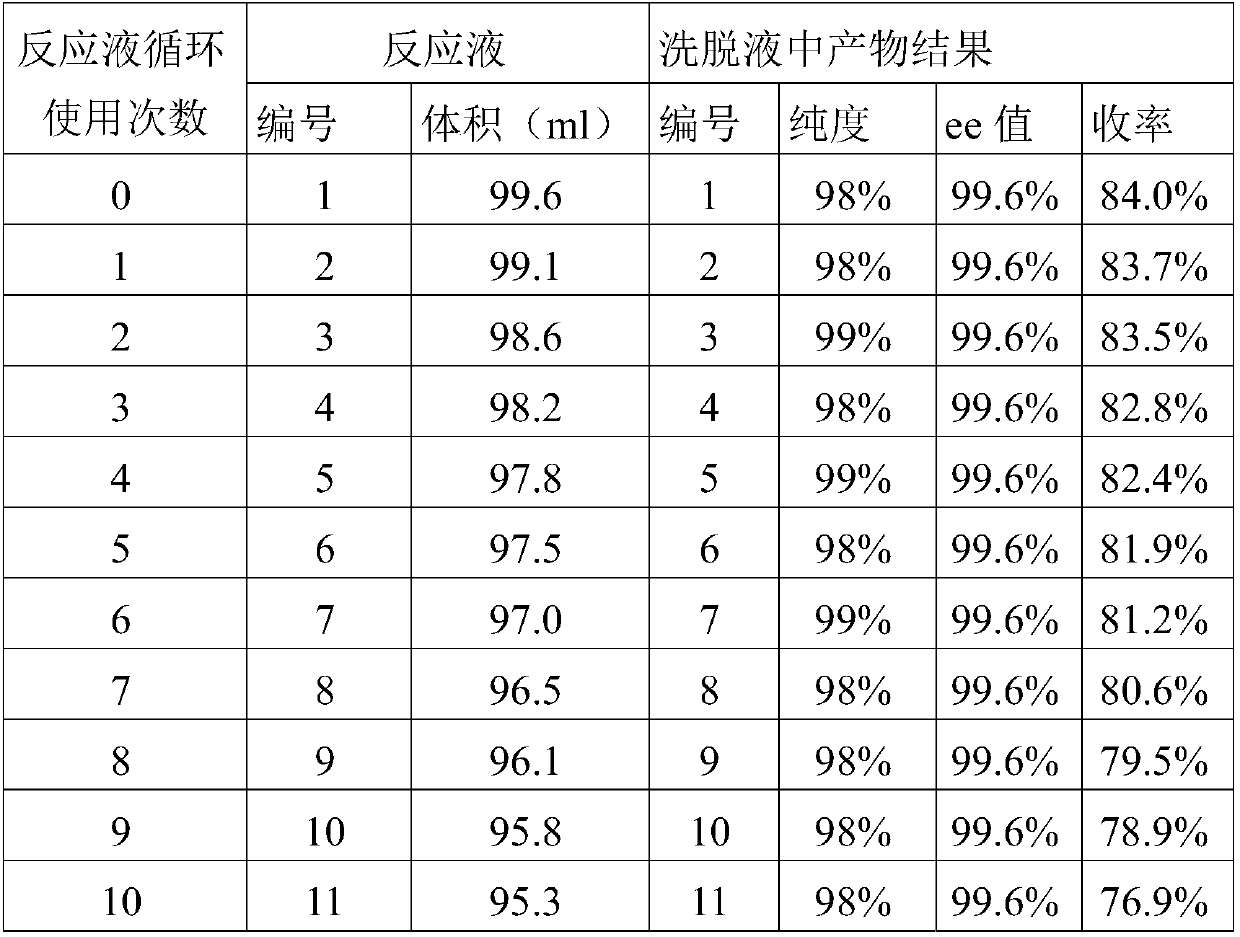
[0076] Embodiment 2 only replenishes the lost volume after each measurement of the collected reaction solution volume, and other implementations are the same as in Example 1, and the resulting eluate product results are shown in Table 2:
[0077] Table 2:
[0078]
[0079]
[0080] Note 1 : The substrate is o-chloromandelonitrile racemate.
[0081] Note 2 : Add new reaction buffer after the volume of the reaction solution decreases.
[0082] Note 3 : Cycle 10 times, and the concentration of substrate catalyzed is equivalent to 1500mM.
[0083] From the experimental results of this example, it can be seen that the yield of the product can be further significantly improved by adding new reaction buffer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com