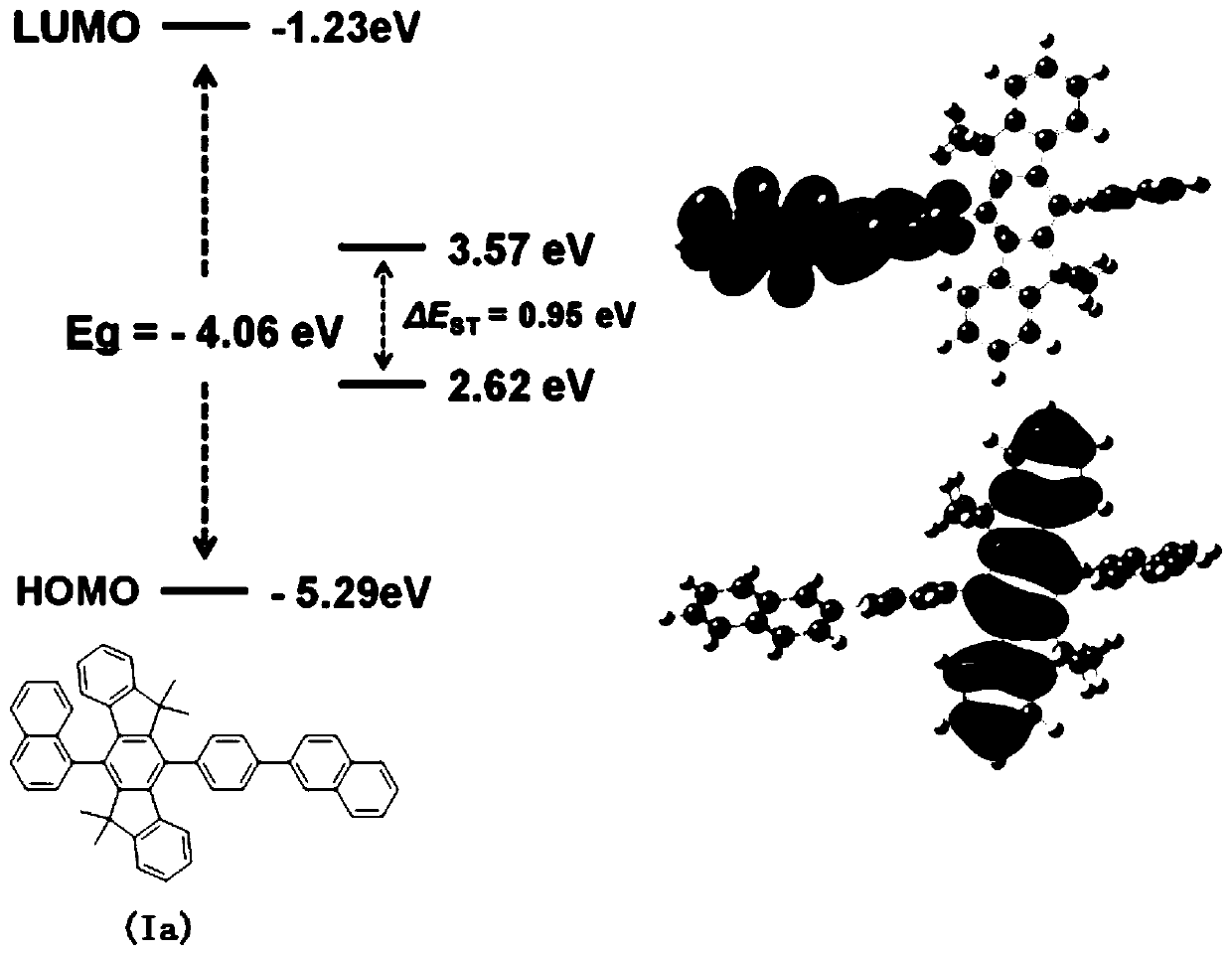
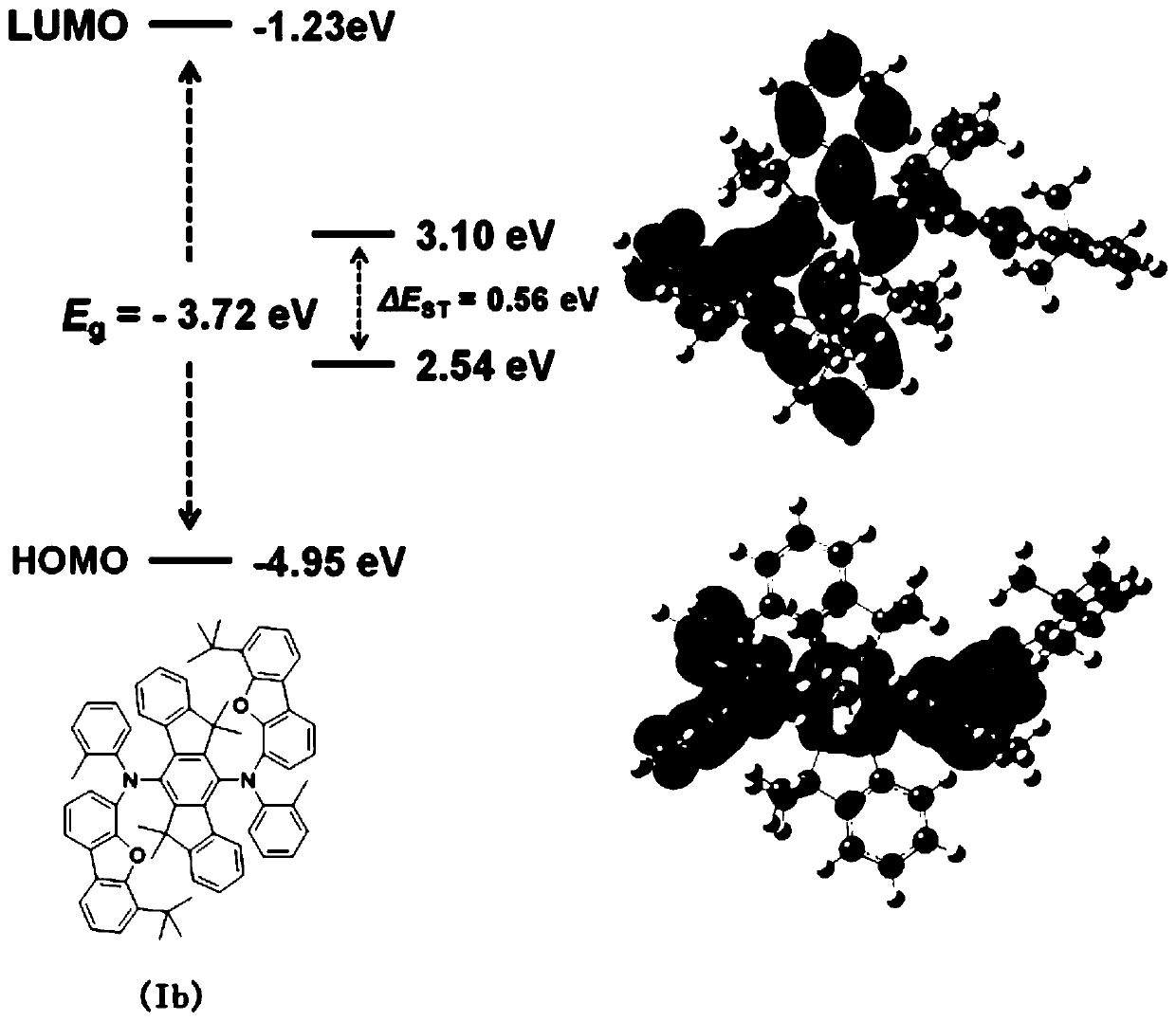
Thickened triphenyl compound as well as preparation method and application thereof
A technology of terphenyl and compound, which is applied in the field of fused terphenyl compound and its preparation, and can solve the problems of shortened service life, low thermal stability, easy annihilation of excitons and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] This embodiment provides a fused terphenyl compound having a structure shown in the following formula (P-009):
[0095]
[0096] The synthetic route of the fused terphenyl compound shown in formula (P-009) is as follows:
[0097]
[0098] Wherein, the synthetic route of the compound shown in formula (C) is as follows:
[0099]
[0100] The concrete of condensed terphenyl compound shown in formula (P-009) comprises the following steps:
[0101] (1) Synthesis of Compound C
[0102] Take a 2-liter double-necked round-bottom bottle and put it into a stirring bar and a reflux tube connected to it, fill it with nitrogen after drying, and add 263.96 grams of compound A (1.0 equivalent) and 892.02 grams of K 2 S 2 o 8 (3.3 equivalents) and 837.57 grams of iodine (3.3 equivalents), add 1000 ml of dichloromethane and cool to 0 ° C, stir and mix for 10 minutes, slowly add a mixed solution of 375 ml of TFA and 17 ml of sulfuric acid, and wrap the double neck with alumi...
Embodiment 2
[0115] This embodiment provides a fused terphenyl compound having a structure shown in the following formula (P-040):
[0116]
[0117] The synthetic route of the fused terphenyl compound shown in formula (P-040) is as follows:
[0118]
[0119] The preparation method of the fused terphenyl compound shown in formula (P-040) specifically comprises the following steps:
[0120] Using the compound shown in formula (C) and phenylboronic acid as raw materials, according to the synthetic method provided in Example 1, the difference is that compound H-1 is replaced by compound H-2 in step (5), to obtain formula Fused terphenyl compound represented by (P-040) (1.065 g, yield 92%).
[0121] Elemental analysis: (C 45 h 38 ) Theoretical value: C, 93.38; H, 6.62; Measured value: C, 93.41; H, 6.59; HRMS (ESI) m / z (M + ): theoretical value: 578.2974; measured value: 578.2889.
Embodiment 3
[0123] This embodiment provides a fused terphenyl compound having a structure shown in the following formula (P-041):
[0124]
[0125] The synthetic route of the fused terphenyl compound shown in formula (P-041) is as follows:
[0126]
[0127] The preparation method of the fused terphenyl compound shown in formula (P-041) specifically comprises the following steps:
[0128] Using the compound shown in formula (C) and phenylboronic acid as raw materials, according to the synthesis method provided in Example 1, the difference is that compound H-1 is replaced by compound H-3 in step (5), to obtain formula Fused terphenyl compound represented by (P-041) (1.050 g, yield 95%).
[0129] Elemental analysis: (C 42 h 32 O) Theoretical value: C, 91.27; H, 5.84; Found value: C, 91.23; H, 5.86; HRMS (ESI) m / z (M + ): theoretical value: 552.2453; measured value: 552.2457.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com