Application of metal nitride, electrolyte containing metal nitride and application of electrolyte in secondary battery
A secondary battery, sodium secondary battery technology, applied in the direction of secondary battery, secondary battery repair/maintenance, organic electrolyte, etc., can solve the problems of few cycles, poor stability, low Coulombic efficiency, etc., and achieve Coulombic efficiency The effect of improving, prolonging the number of cycles, and suppressing lithium dendrites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
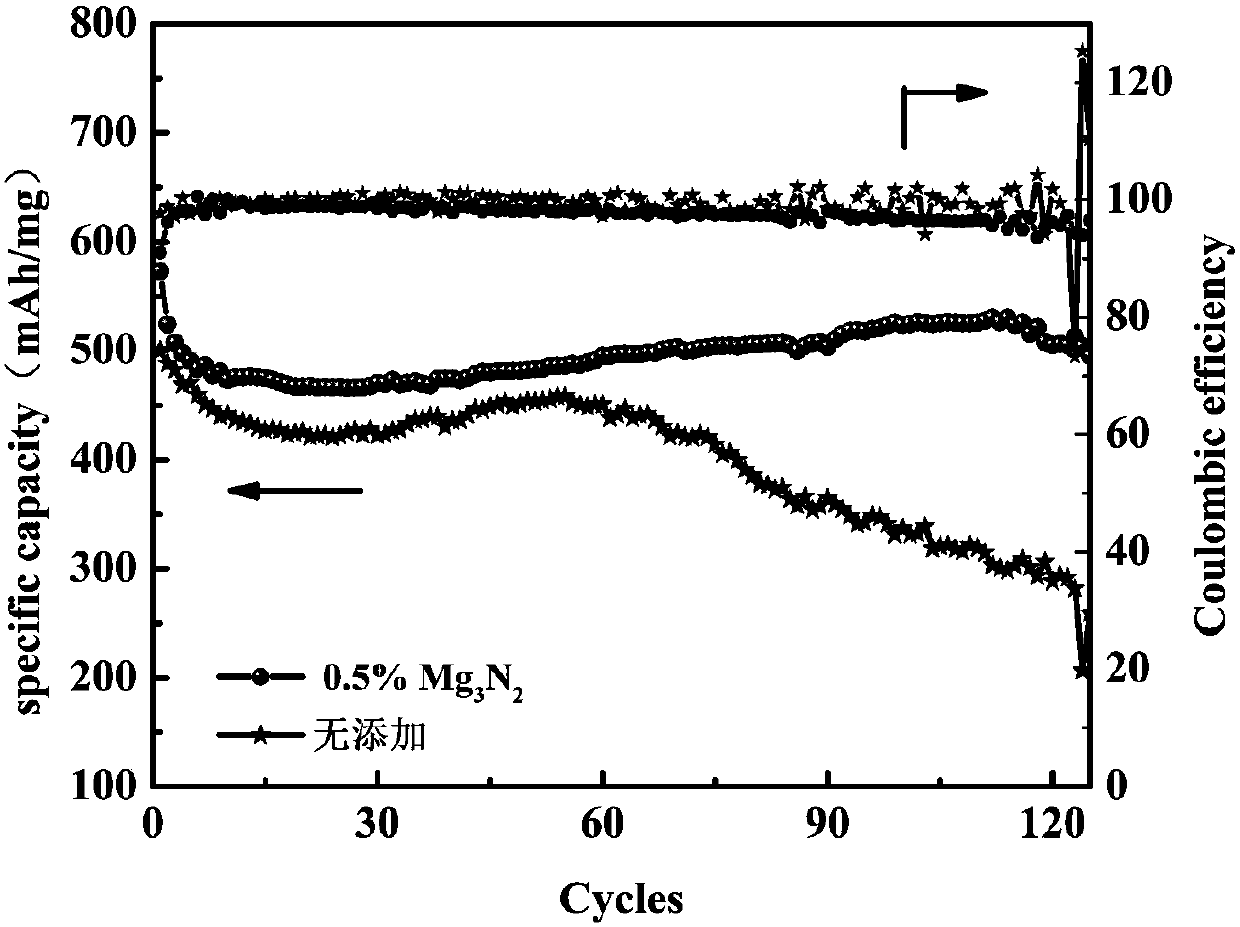
[0058] In the above electrolyte 1M LiTFSI / DOL:DME (1:1Vol%), add 0.5wt.%Mg 3 N 2 As an additive, the electrolyte assembled battery without adding magnesium nitride is used as a comparison sample, and the cycle life and Coulombic efficiency graph at 0.5C rate ( figure 1 ). Cycle at 0.5C with addition of Mg 3 N 2 The battery performance of the electrolyte is significantly better than that of the control sample, and after 60 cycles, the control sample begins to decline significantly, while the battery containing the additive can still continue to cycle stably up to 120 cycles. The addition of nitrides has obvious beneficial effects.
Embodiment 2
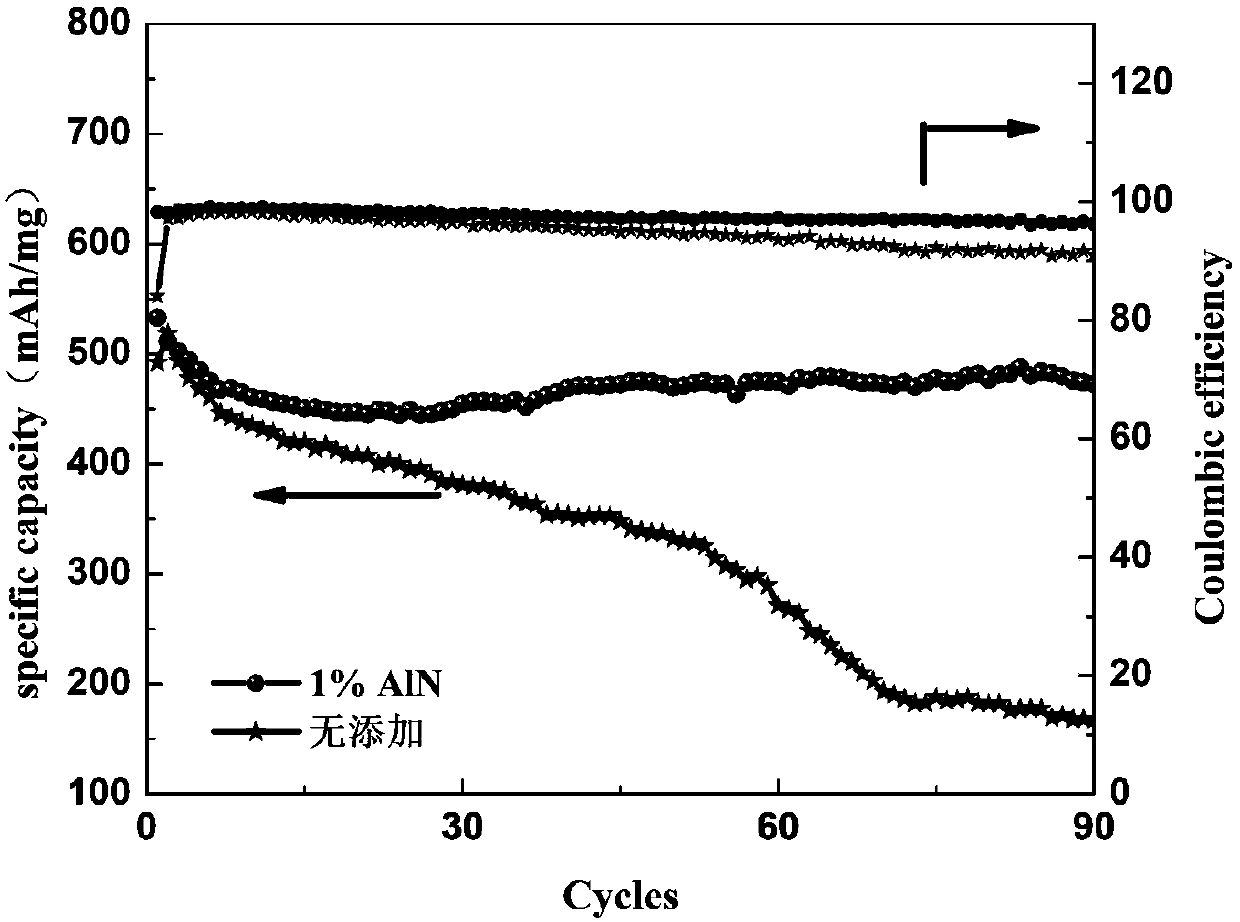
[0060] In the above electrolyte 1M LiTFSI / DOL:DME (1:1Vol%), add 1Wt.% AlN as an additive, and use the electrolyte assembled battery without adding magnesium nitride as a comparison sample, the cycle life and Coulombic efficiency diagram ( figure 2 ). The battery assembled with the electrolyte added with AlN can be cycled stably for 90 cycles, while the cycle capacity of the control battery keeps decreasing and cannot continue to cycle stably. The effect of nitride addition is obvious.
Embodiment 3
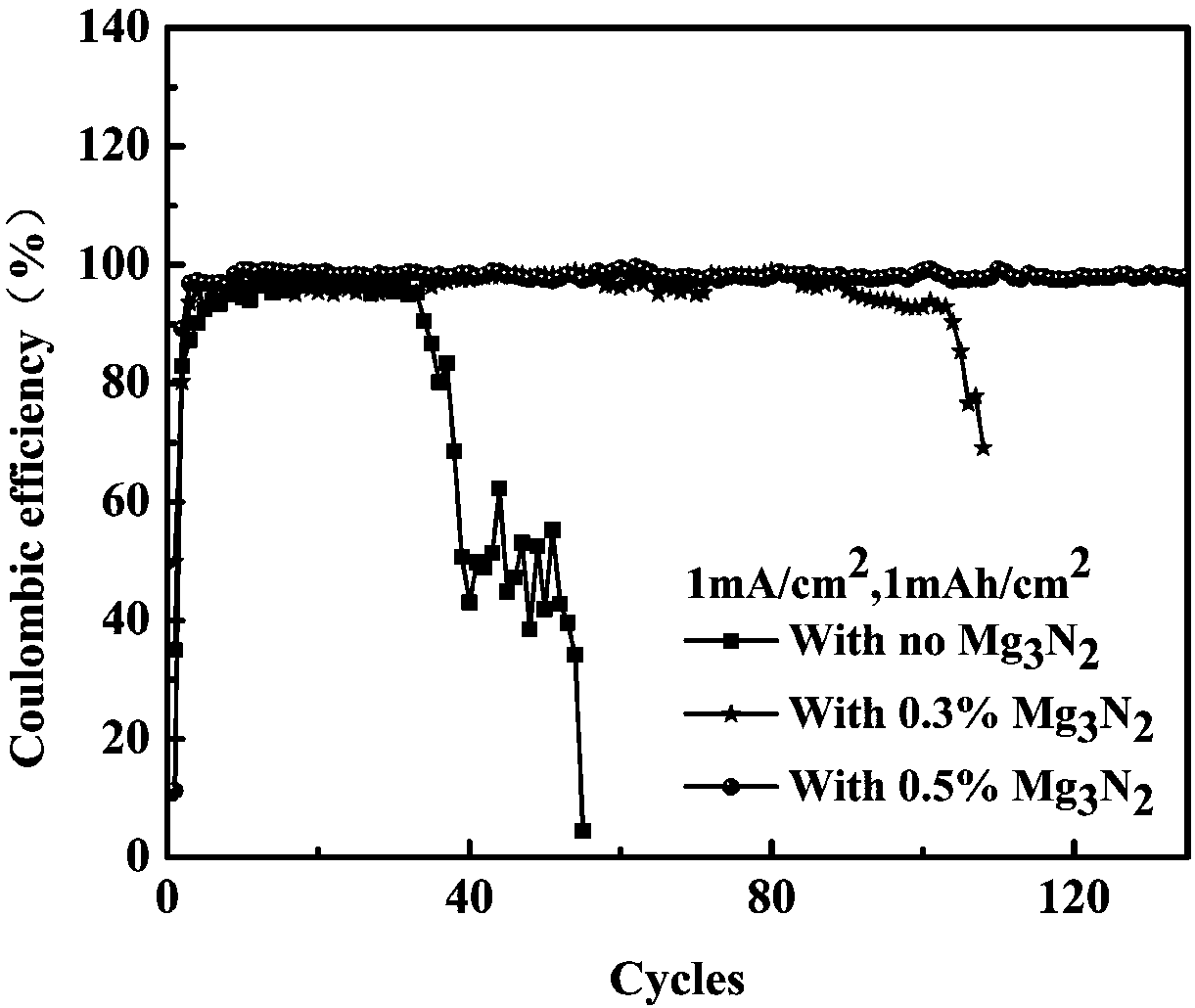
[0062] Add mass fractions of 0.3% and 0.5% Mg to the above electrolyte 1M LiTFSI / DOL:DME (1:1Vol%) 3 N 2 , after assembling the half-cell, at a charge density of 1mA / cm 2 , the power is 1mAh / cm 2 The next cycle, see the results image 3 . Add Mg 3 N 2 The beneficial effect of 0.5% of the electrolyte on the half-cell can reach more than 130 cycles, the beneficial effect of the electrolyte added at 0.3% on the half-cell can reach about 90 cycles, and the addition of different amounts of Mg 3 N 2 Both are significantly improved compared to the non-additive half-cell. But the effect of adding more than 0.5% is better.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com