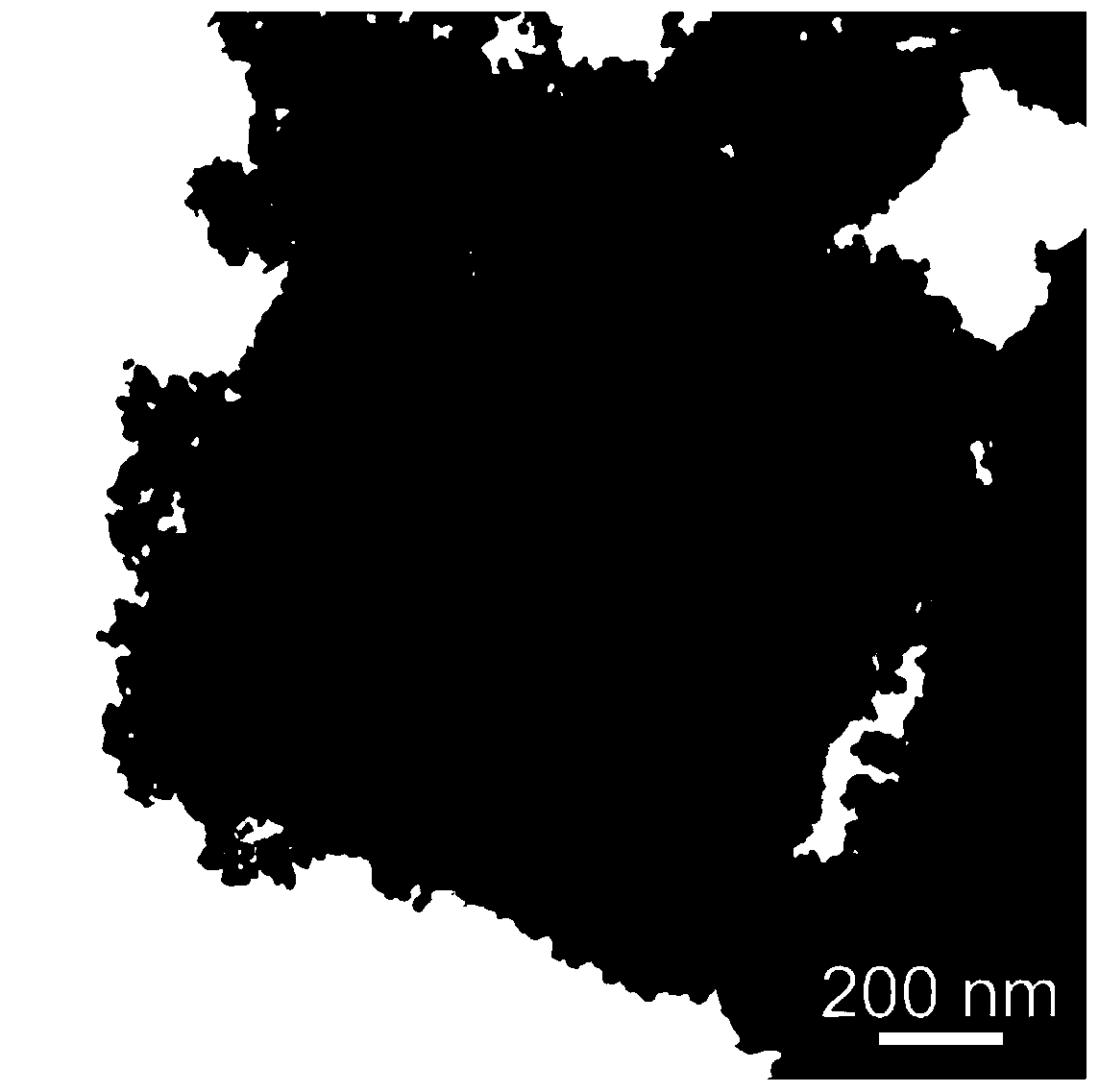
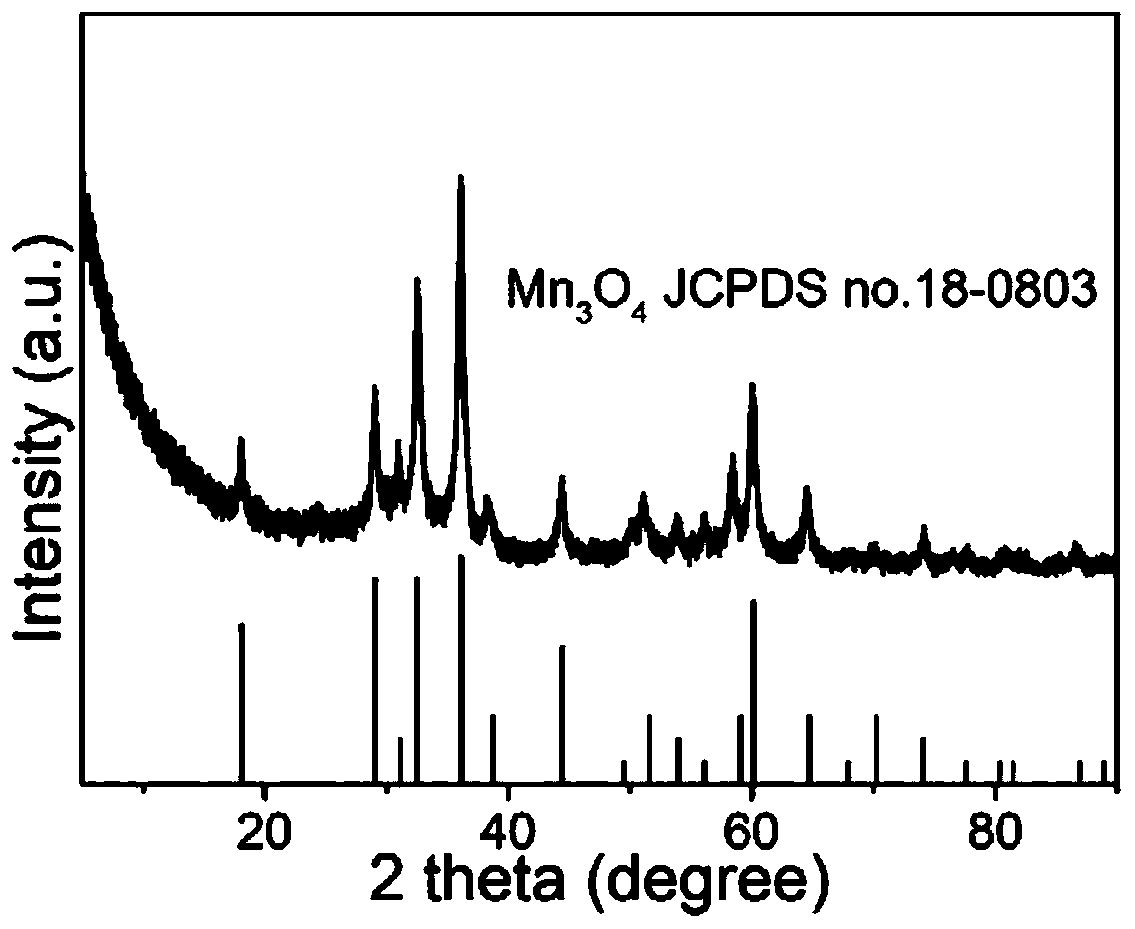
Method for preparing Fe-doped Mn3O4 carbon-nitrogen material with hollow nano-frame structure and application thereof
A frame structure, carbon-nitrogen material technology, applied in the direction of structural parts, electrical components, battery electrodes, etc., can solve the problems of time-consuming, cumbersome preparation process, and low output, and achieve difficult agglomeration, high oxygen reduction activity, and good morphology regular effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A Fe-doped Mn with hollow nanoframe structure 3 o 4 The preparation method of carbon nitrogen material, comprises the following steps:
[0042] 1)KMnFe(CN) 6 Preparation of khaki precipitate: weigh 3.0g of PVP, 1.0mmol of K 3 Fe(CN) 6 ·3H 2 O solid metal salt and 50ml H 2 The O solution was mixed and mechanically stirred for 10 min at room temperature to obtain Fe(CN) 6 3- / PVP solution; Weigh 1.5mmol of MnCl 2 with 50ml H 2 The O solution was mixed and mechanically stirred for 10 min at room temperature to obtain Mn 2+ solution; the Fe(CN) 6 3- / PVP solution and Mn 2+ The solutions were mixed and allowed to stand for 12 hours to obtain KMnFe(CN) 6 Earthy yellow precipitate;
[0043] 2) Preparation of Fe-doped Mn with hollow nanoframe structure 3 o 4 Carbon nitrogen material: the KMnFe(CN) prepared in step 1) 6 The khaki precipitate was washed with alkali in 0.2 M NaOH solution at 40 ° C for 4 h, and the obtained solid powder material was first washed a...
Embodiment 2
[0046] A Fe-doped Mn with hollow nanoframe structure 3 o 4 The preparation method of carbon nitrogen material, comprises the following steps:
[0047] 1)KMnFe(CN) 6 Preparation of khaki precipitate: weigh 3.5g of PVP, 1.0mmol of K 3 Fe(CN) 6 ·3H 2 O solid metal salt and 50ml H 2 The O solution was mixed and mechanically stirred for 10 min at room temperature to obtain Fe(CN) 6 3- / PVP solution; Weigh 1.5mmol of MnCl 2 with 50ml H 2 The O solution was mixed and mechanically stirred for 10 min at room temperature to obtain Mn 2+ solution; the Fe(CN) 6 3- / PVP solution and Mn 2+ The solutions were mixed and allowed to stand for 12 hours to obtain KMnFe(CN) 6 Earthy yellow precipitate;
[0048] 2) Preparation of Fe-doped Mn with hollow nanoframe structure 3 o 4 Carbon nitrogen material: the KMnFe(CN) prepared in step 1) 6 The khaki precipitate was washed with alkali in 0.2 M NaOH solution at 40 ° C for 4 h, and the obtained solid powder material was first washed a...
Embodiment 3
[0050] A Fe-doped Mn with hollow nanoframe structure 3 o 4 The preparation method of carbon nitrogen material, comprises the following steps:
[0051] 1)KMnFe(CN) 6 Preparation of khaki precipitate: weigh 4.0g of PVP, 1.0mmol of K 3 Fe(CN) 6 ·3H 2 O solid metal salt and 50ml H 2 The O solution was mixed and mechanically stirred for 10 min at room temperature to obtain Fe(CN) 6 3- / PVP solution; Weigh 1.5mmol of MnCl 2 with 50ml H 2 The O solution was mixed and mechanically stirred for 10 min at room temperature to obtain Mn 2+ solution; the Fe(CN) 6 3- / PVP solution and Mn 2+ The solutions were mixed and allowed to stand for 12 hours to obtain KMnFe(CN) 6 Earthy yellow precipitate;
[0052] 2) Preparation of Fe-doped Mn with hollow nanoframe structure 3 o 4 Carbon nitrogen material: the KMnFe(CN) prepared in step 1) 6 The khaki precipitate was washed with alkali in 0.2 M NaOH solution at 40 ° C for 4 h, and the obtained solid powder material was first washed a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com