Piperazinone derivatives, preparation method thereof, inhibitor, and method for preventing and controlling root parasitic weeds
The technology of a derivative, piperazinone, is applied in the field of pesticides, which can solve the problems of unstable ingredients and high cost of compounds, and achieve the effects of stable ingredients, low preparation costs, and inhibition of germination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
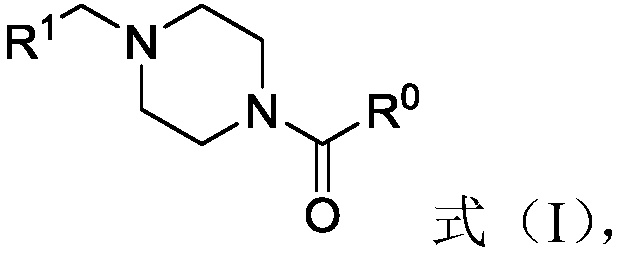
[0033] The second aspect of the present invention provides a method for preparing piperazinone derivatives, comprising the following steps:
[0034] (A) In the presence of the first solvent and reducing agent, the R 1 -CHO and tert-butoxycarbonylpiperazine carry out reductive amination reaction to obtain synthetic amine; wherein, R 1 is cyclohexenyl, norbornyl, norbornenyl or adamantyl;
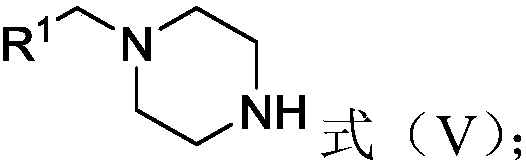
[0035] (B) in the presence of a second solvent and an acid, subjecting the synthetic amine to a tert-butoxycarbonyl removal reaction to obtain an intermediate represented by formula (V), Formula (V);
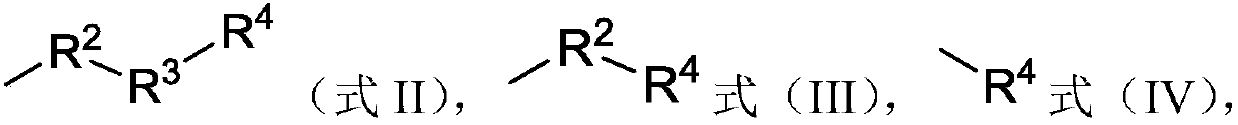
[0036] (C) in the presence of a third solvent, a base and an optional condensing agent, subjecting the intermediate to a compound having a structure shown in formula (VI), formula (VII), formula (VIII) or formula (IX) condensation reaction, Formula (VI), Formula (VII), Formula (VIII), Formula (IX),
[0037] Wherein, Z is hydroxyl, chlorine atom or bromine atom; R 2 is methylene, met...
Embodiment 1
[0076] (A) Preparation of intermediate C: 1-(cyclohexane-3-en-1-ylmethyl)piperazine
[0077]
[0078] 3-cyclohexene-1-carbaldehyde (represented by A, 5g, 45.5mmol), tert-butoxycarbonylpiperazine (represented by B, 8.46g, 45.5mmol) and dichloromethane (150mL) were added to the reaction flask , and cooled to 0°C. Sodium triacetoxyborohydride (14.45 g, 68.2 mmol) was added under stirring, and after 30 min, the system was moved to room temperature and the reaction was stirred overnight. After the reaction was completed, water (100 mL) was added to the system and vigorously stirred for 15 min. Separate the organic layer, wash the aqueous layer with 50 mL of dichloromethane, combine the organic layers, wash the organic layer with water and saturated brine successively, dry with anhydrous sodium sulfate, remove the organic solvent under reduced pressure, and the obtained synthetic amine is not After separation, it was directly used in the next reaction, wherein the yield of the ...
Embodiment 2
[0087] (A) Preparation of intermediate C: 1-(cyclohexan-3-en-1-ylmethyl)piperazine. Prepared according to the method of Example 1.
[0088] (B) Preparation of Compound I-2: 1-(4-(3-cyclohexene-1-methylene)piperazin-1-yl)-2-(2,4-dichlorophenoxy)ethanone
[0089]
[0090] 1-(3-cyclohexene-1-methylene)piperazine (expressed as C, 0.2g, 1.11mmol), 2-(7-oxybenzotriazole)-N,N,N', N'-tetramethyluronium hexafluorophosphate (HATU, 0.55g, 1.44mmol), 2,4-dichlorophenoxyacetic acid (expressed as E, 0.25g, 1.11mmol) and dichloromethane (30mL) were added to in the reaction vial. N,N-Diisopropylethylamine (DIPEA, 0.287g, 2.22mmol) was added dropwise under stirring and the reaction was stirred at room temperature for 10h. After the reaction was completed, 20 mL of water was added to the system and vigorously stirred for 10 min. The organic layer was separated, the aqueous layer was extracted once again with 20mL of dichloromethane, the organic layers were combined, the organic layer was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com