Multi-fluorescent PCR kit and method for detecting clostridium difficile genes and toxin genes
A technology of multiple fluorescence and Clostridium difficile, which is applied in biochemical equipment and methods, recombinant DNA technology, microbial determination/inspection, etc., can solve the problem of unsatisfactory accuracy and sensitivity of CD detection, accuracy, stability and sensitivity Low, limited application and other issues to achieve the effect of avoiding false positive results, avoiding PCR false negatives, and short reporting time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A multiplex fluorescent PCR kit for detecting Clostridium difficile genes and toxin genes, including a set of multiplex fluorescent PCR primers and probes for detecting Clostridium difficile genes and toxin genes, nucleic acid extraction reagents, and multiplex fluorescent PCR reactions Required reagents, internal standard working solution, positive control solution and negative control solution.
[0049] Wherein, the multiple fluorescent PCR primer sets and probes for detecting Clostridium difficile gene and toxin gene include tpi amplification primer pair tpi-F and tpi-R, tpi fluorescent probe tpi-P, tcdB amplification primer For tcdB-F and tcdB-R, tcdB fluorescent probe tcdB-P, internal standard primer pair HP-F and HP-R, internal standard fluorescent probe HP-P; the nucleotide sequences are respectively as SEQID NO:1 ~shown in SEQ ID NO:9.
[0050] The fluorescent substances bound to the 5' ends of the probes tpi-P, tcdB-P, and HP-P are FAM, HEX, and Cy5 respective...
Embodiment 2
[0073] The detection of the clinical sample by the kit of embodiment 2
[0074] Using the kit of Example 1 to detect Clostridium difficile in clinical samples, the specific steps are as follows:
[0075] 1. Sample settings
[0076]When collecting human feces samples, the samples should not be mixed with urine, urinal water, or disinfectants, and should not touch urinals, toilet paper, diapers, etc., to prevent the samples from being contaminated or damaged. Set 10 Clostridium difficile positive clinical samples (P1-P10) and 10 Clostridium difficile negative clinical samples (N1-N10), wherein positive samples include Clostridium difficile toxin-producing strains (P1-P5) and non-toxin-producing strains ( P6-P10). All clinical stool samples were isolated and cultured, identified by mass spectrometry, and verified by Sanger sequencing of toxins in the Laboratory Department of Guangzhou Military General Hospital.
[0077] 2. Extraction of Fecal Genomic DNA
[0078] (1) Pick 50-...
Embodiment 3
[0113] Embodiment 3 kit performance analysis
[0114] 1. Specificity analysis
[0115] (1) Cross reaction
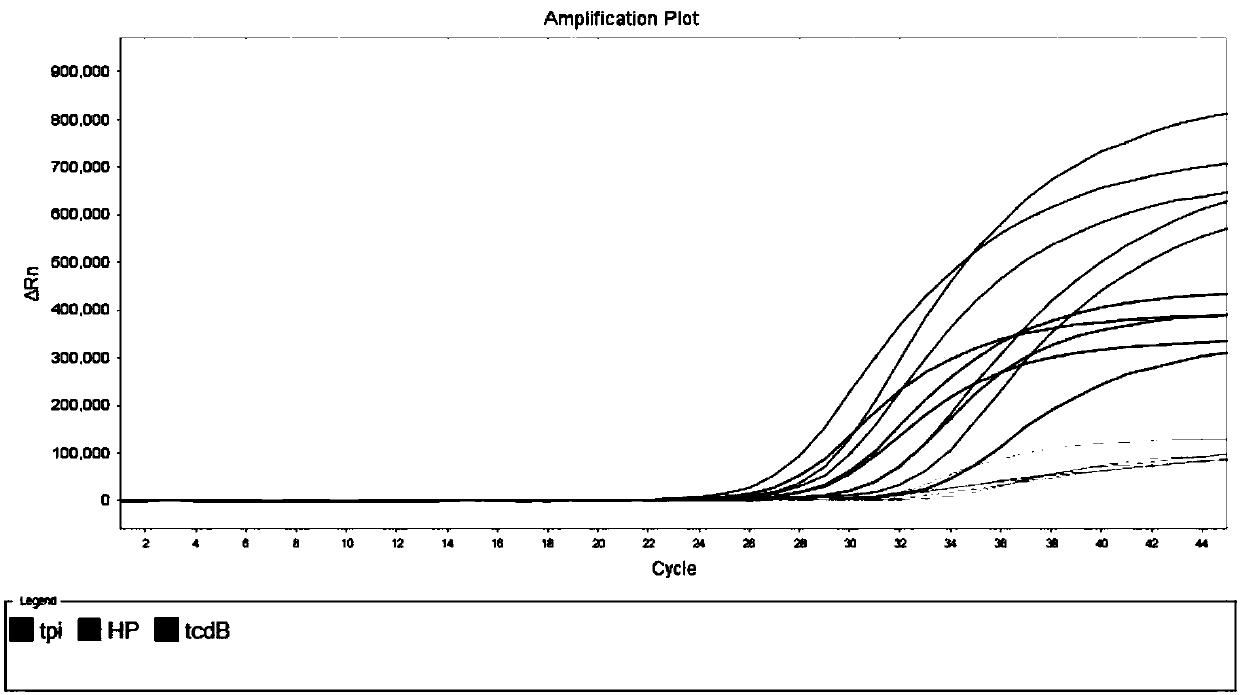
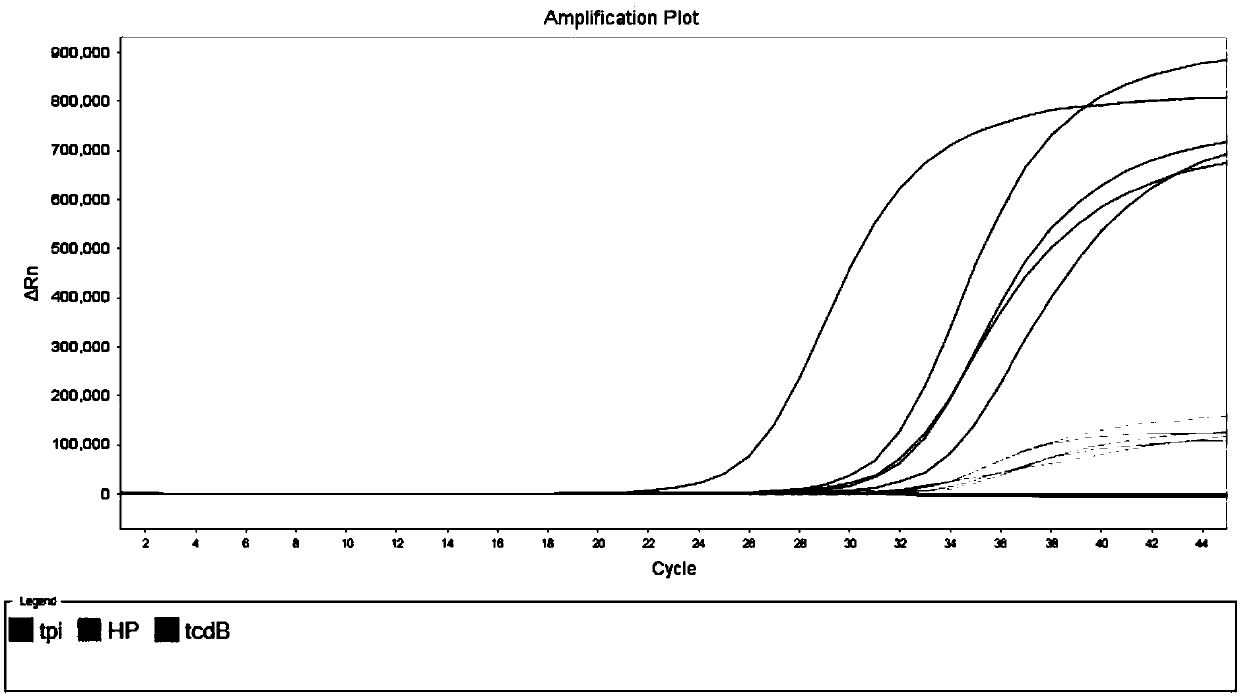
[0116] Using the kit described in Example 1 and the detection method described in Example 2, select microorganisms that are similar to Clostridium difficile or cause similar symptoms, such as Yersinia enterocolitica, enterotoxic Escherichia coli and cereus 21 species such as Bacillus were samples to be tested, and the coincidence rate of the kit in Example 1 for detecting other pathogens similar to Clostridium difficile and causing similar symptoms was evaluated to analyze the specificity of the kit. The concentration of the sample to be tested is estimated after measuring the OD value, which is higher than 1×10 6 bacteria / mL, the results are shown in Table 7 and Figure 5 shown.
[0117] Experimental results: In addition to the amplification of the internal standard, no amplification peaks were detected for the tpi and tcdB targets, indicating that none of these sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com