Reagent kit of detecting human immunodeficiency virus type 1 by fluorescence quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction)
A human immunodeficiency, fluorescent quantitative technology, applied in the field of fluorescent quantitative RT-PCR detection kits for human immunodeficiency virus type 1, can solve problems such as incurable, no effective vaccine, etc., to reduce false negative results and avoid false negatives and false positives, improving reliability and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Detection method of human immunodeficiency virus type 1 kit
[0039] (1) Specimen collection, transportation and preservation
[0040] Specimen collection:
[0041] Serum-Use a disposable sterile syringe to draw 2 ml of the subject's venous blood, inject it into a sterile dry glass tube, and place it at room temperature (22~25℃) for 30~60 minutes. The blood sample can spontaneously and completely agglutinate and separate the serum, or use it directly Centrifuge at 1500rpm for 5 minutes in a horizontal centrifuge; aspirate the upper layer of serum and transfer it to a 1.5ml sterile centrifuge tube.
[0042] Plasma-Use a disposable sterile syringe to draw 2 ml of the subject's venous blood, and inject it into a glass tube containing EDTA-2Na (disodium ethylenediaminetetraacetic acid) or sodium citrate anticoagulant, and then gently invert the glass tube to mix immediately 5-10 times, fully mix the anticoagulant and venous blood. After 5-10 minutes, the plasma can be s...
Embodiment 2
[0081] Example 2: Use of quality control materials and positive quantitative reference materials in the human immunodeficiency virus type 1 kit
[0082] The quality control materials and positive quantitative reference materials in the human immunodeficiency virus type 1 kit include HIV-1 strong positive quality control materials, HIV-1 borderline positive quality control materials, negative control materials and HIV-1 positive quantitative reference materials 1- 4. Used for quality control in clinical trials, the operation method is the same as the sample to be tested.
[0083] Quality Control:
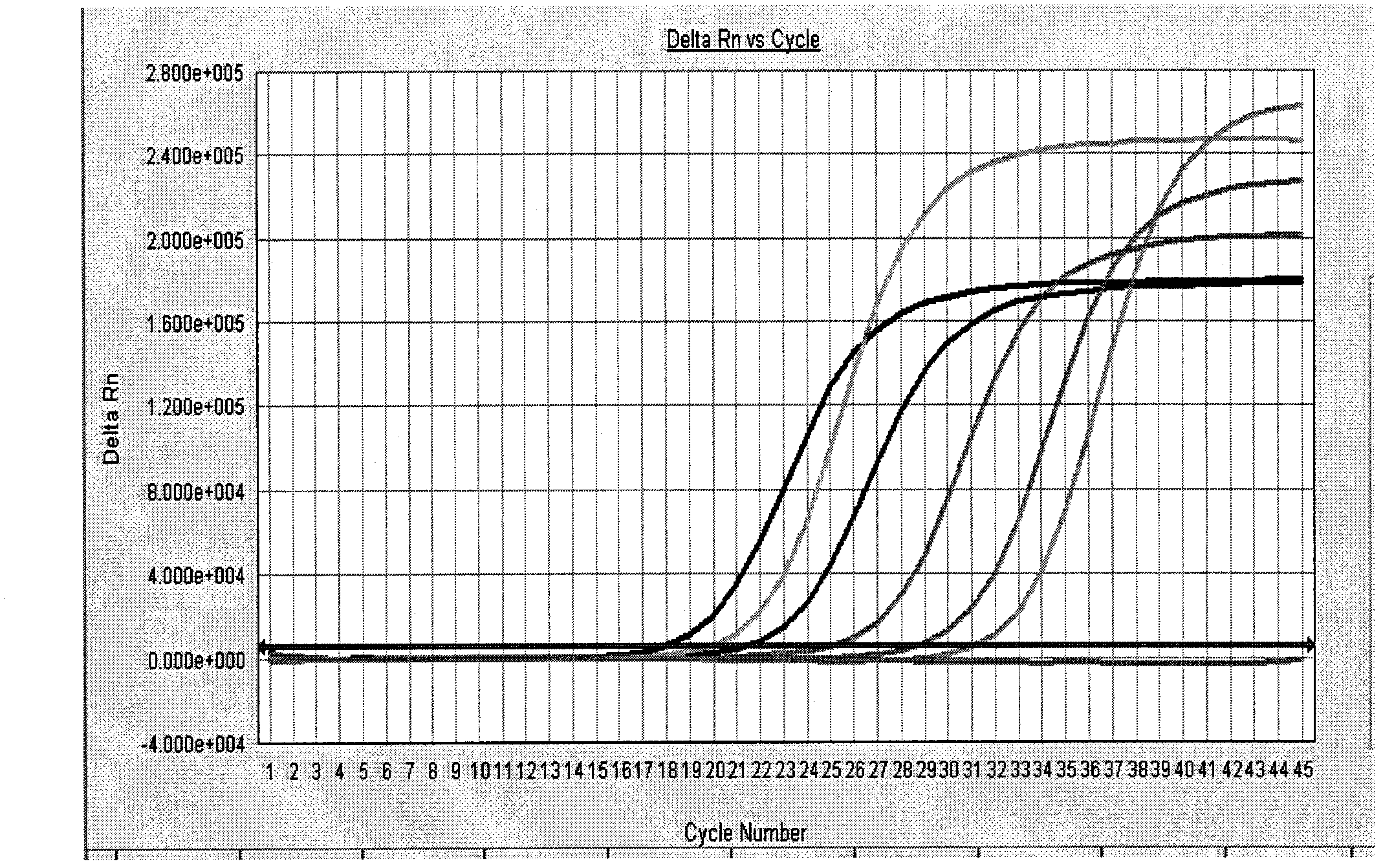
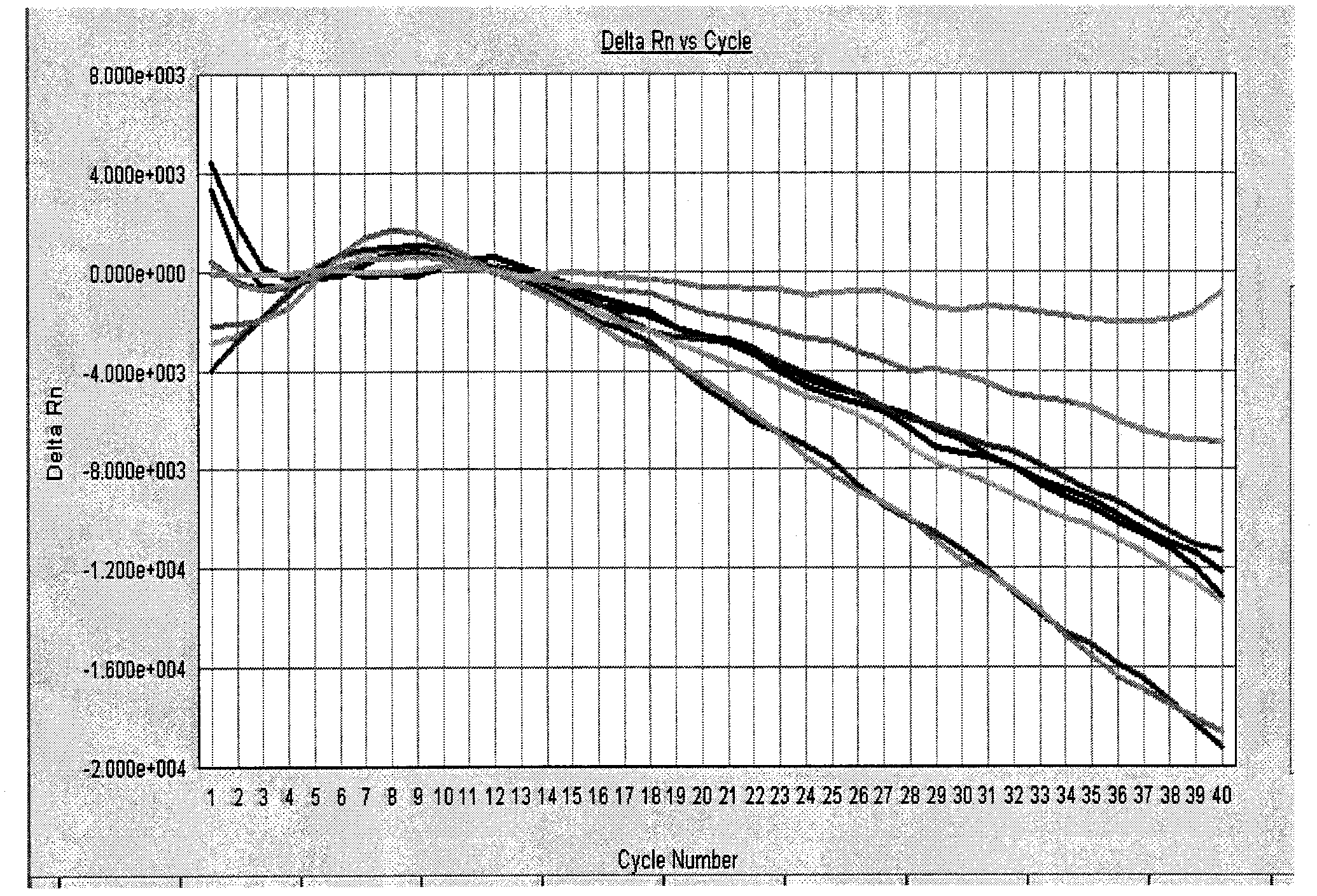
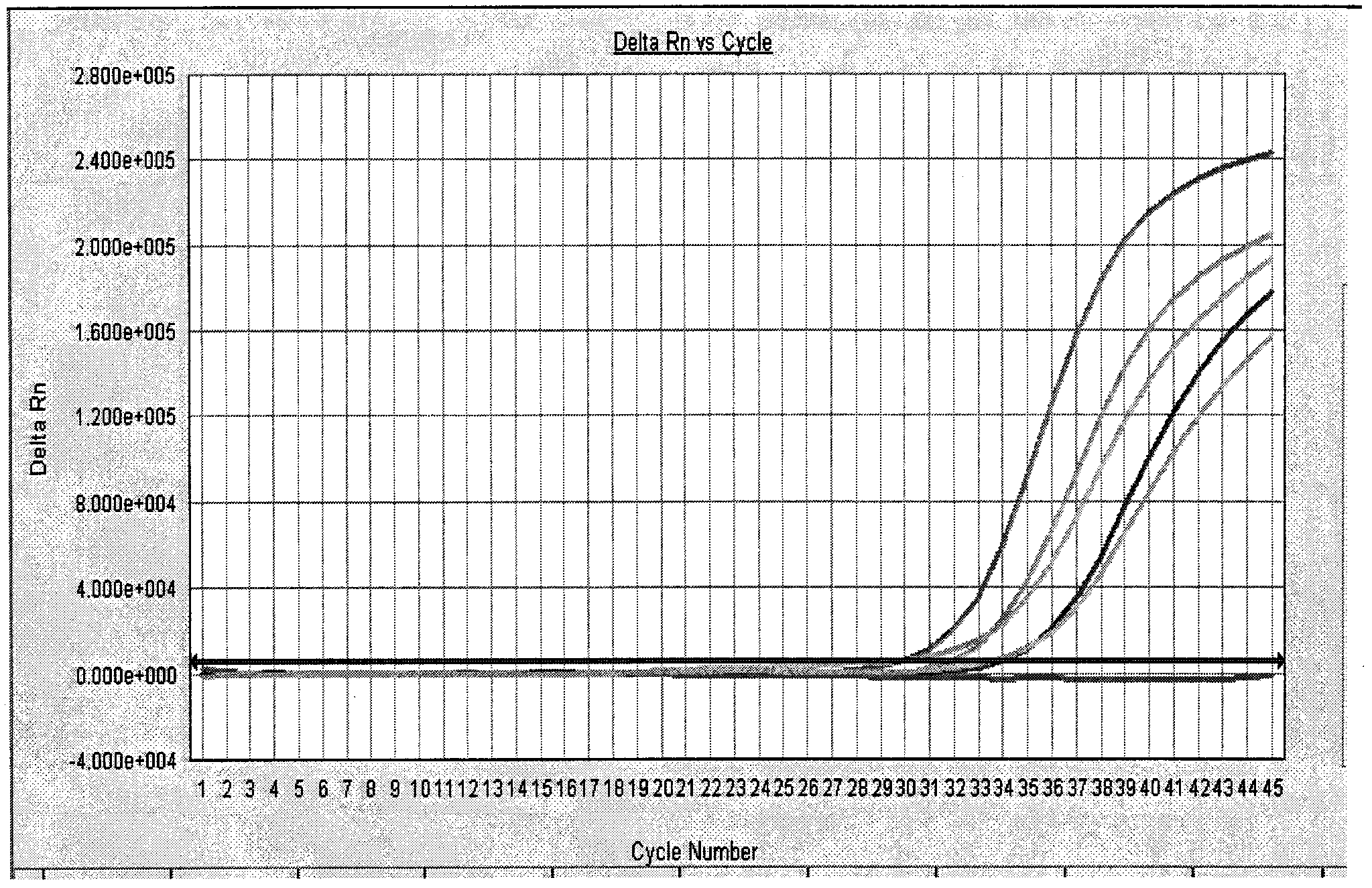
[0084] Negative control: FAM detection pathway amplification curve has no logarithmic growth period, VIC detection pathway amplification curve is logarithmic growth period;
[0085] HIV-1 positive quality control products: the FAM detection pathway amplification curve has a significant logarithmic growth period, showing a typical S-type amplification curve, and the HIV-1 strong positive qual...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com