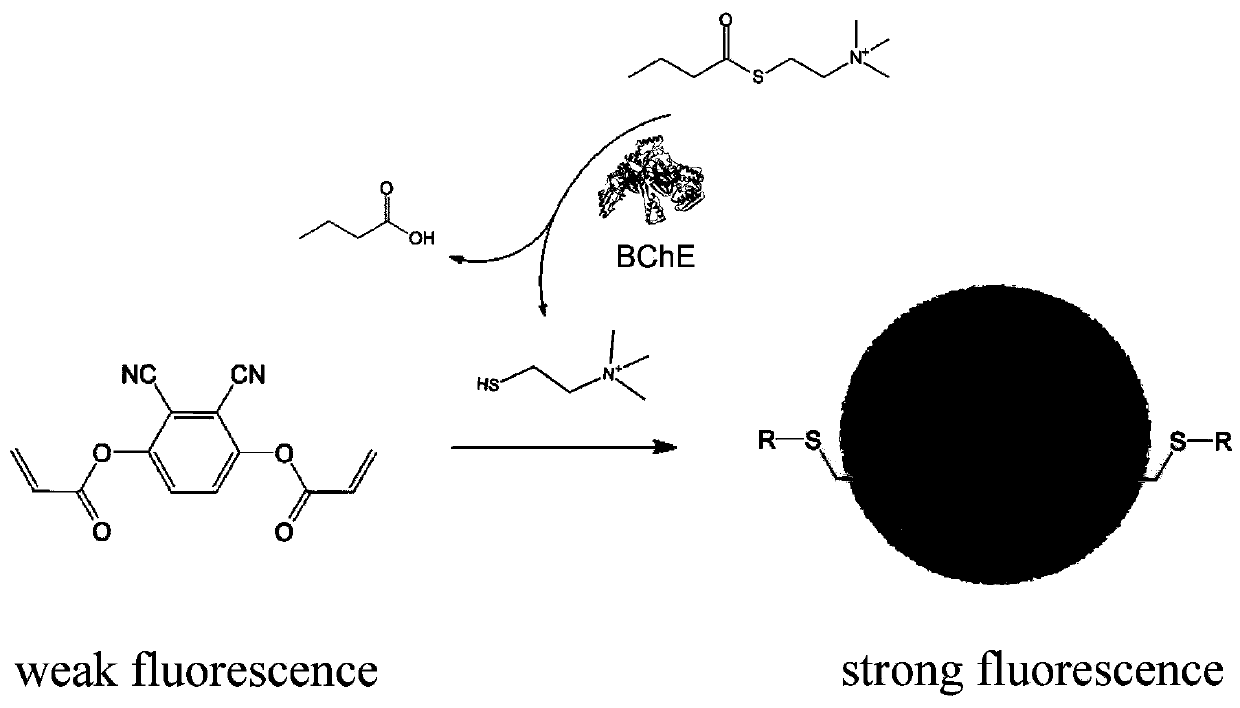
Fluorescent probe for detecting activity of butyrylcholineesterase, preparation method and application thereof
A technology of butyrylcholinesterase and fluorescent probes, applied in the field of fluorescent probes, can solve problems such as no public detection function, achieve good sensitivity and avoid interference effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Synthesis of 2,3-dicyano-1,4-phenylene diacrylate (DCPDA for short).
[0040] The steps of synthesizing DCPDA are as follows:
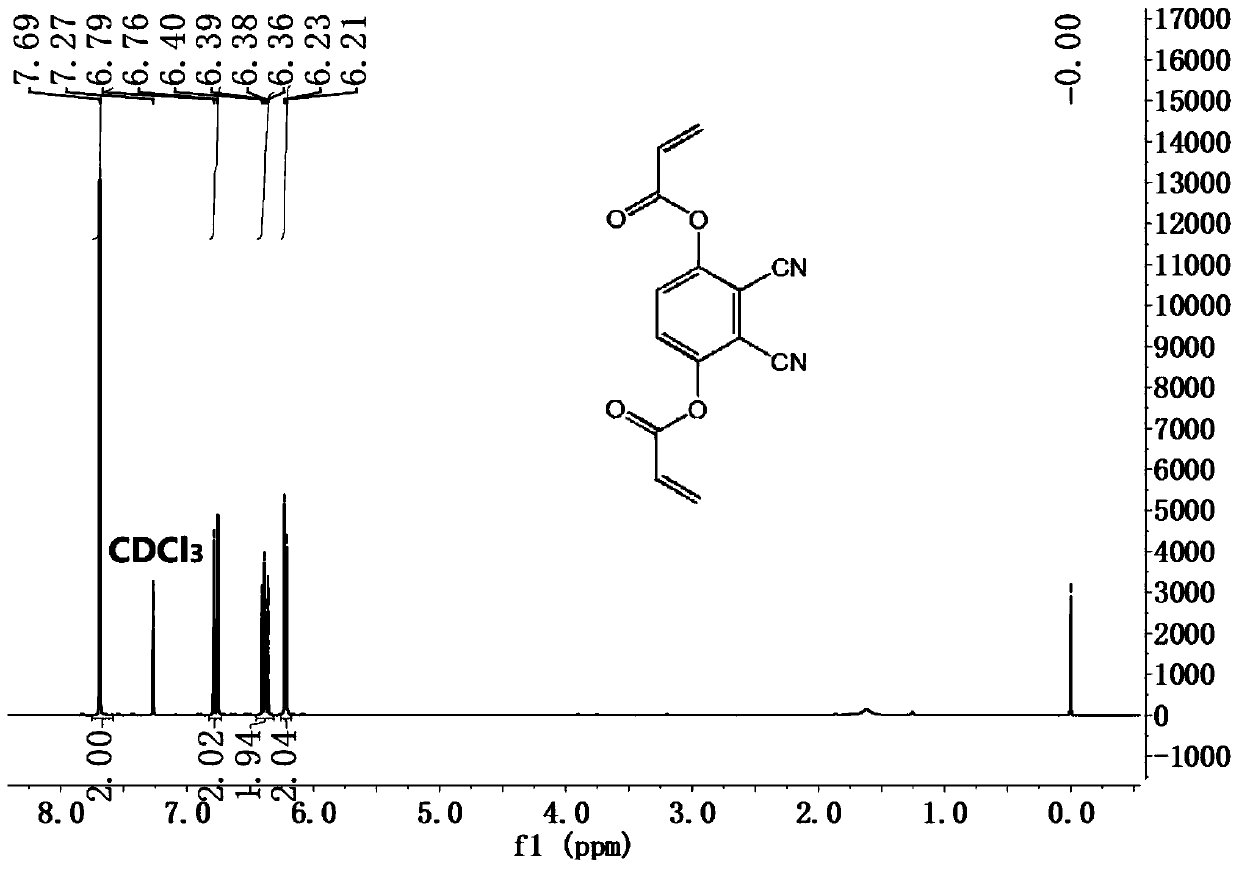
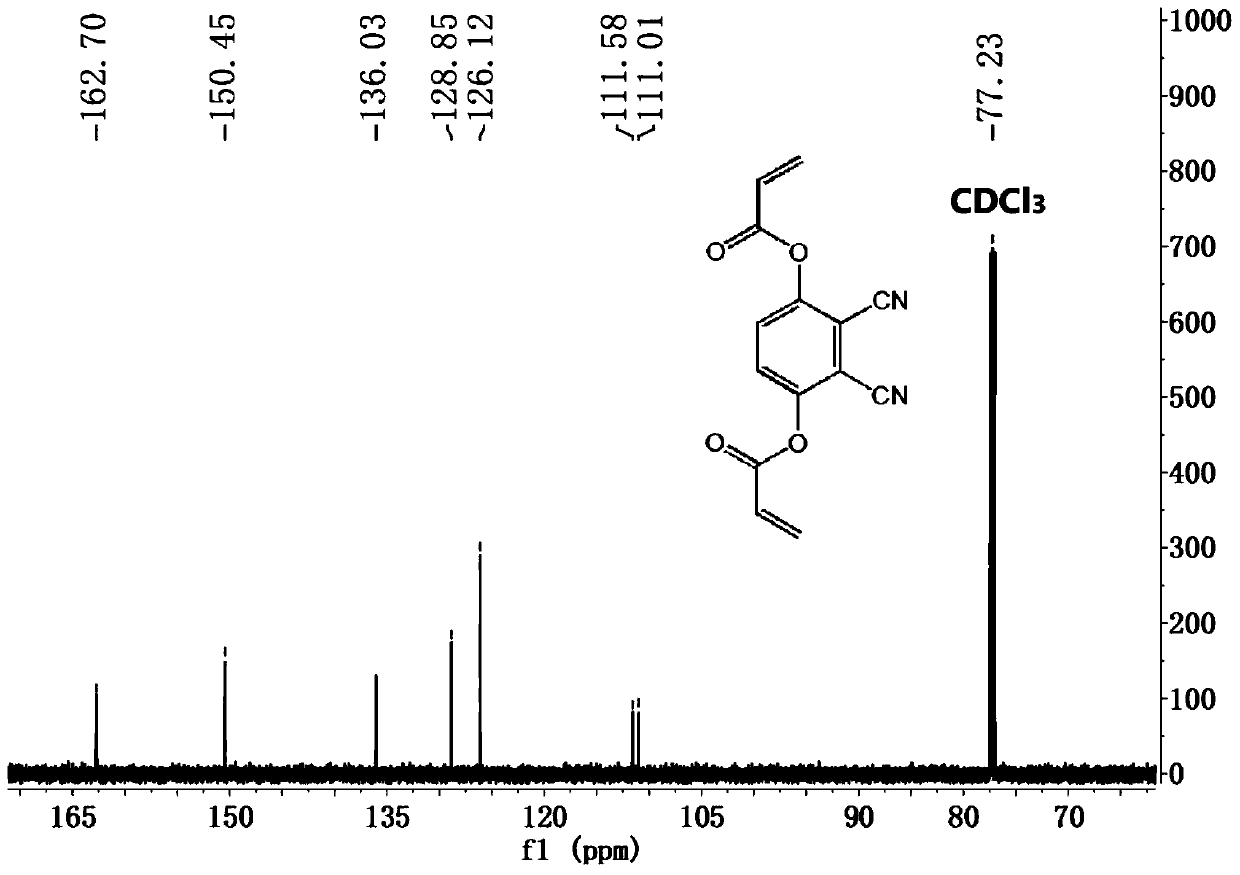
[0041] First, 0.5 g (ie 3.1 mmol) of 2,3-dicyanohydroquinone was dissolved in 50 mL of dry THF, and then 2.0 mL of excess acryloyl chloride was added dropwise. After adding a small amount of triethylamine, the resulting mixture was stirred well for 24 hours. Finally, the solvent was removed by a rotary evaporator, and the product was purified by silica gel column chromatography (ethyl acetate:petroleum ether 1:4). The final product DCPDA is a white powder with a yield of 36%. The NMR spectrum and high-resolution mass spectrum of the DCPDA and the structural formula of DCPDA are detailed in Figure 2 to Figure 4 shown.
[0042] The reaction formula of 2,3-dicyanohydroquinone and acryloyl chloride is as follows:
[0043] R-COCl+R'OH→RCOOR'+HCl
[0044] 2. The fluorescence response of 2,3-dicyano-1,4-phenylene diacrylate (DCPDA) to mercapto ...
Embodiment 2
[0063] 1. Synthesis of 2,3-dicyano-1,4-phenylene bis(3,3-dimethacrylate) (DCPDMA).
[0064] Synthetic DCPDMA steps are as follows:
[0065] First, 0.5 g (substance amount: 3.1 mmol) of 2,3-dicyanohydroquinone was dissolved in 50 mL of dry THF, and then 2.0 mL of excess 3,3-dimethylacryloyl chloride was added dropwise. After adding a small amount of triethylamine, the resulting mixture was stirred well for 24 hours. Finally, the solvent was removed by a rotary evaporator, and the product was purified by silica gel column chromatography (ethyl acetate:petroleum ether 1:4). The final product was a white powder with a yield of 36%.
[0066] The reaction formula of 2,3-dicyanohydroquinone and 3,3-dimethylacryloyl chloride is as follows:
[0067] R-COCl+R'OH→RCOOR'+HCl
[0068] 2. The fluorescence response of 2,3-dicyano-1,4-phenylene bis(3,3-dimethacrylate) (DCPDMA) to mercapto compounds.
[0069] Add a certain amount of DCPDMA solution into 3.0mL HEPES buffer solution, wherei...
Embodiment 3
[0085] 1. Synthesis of 2,3-dicyano-1,4-phenylene bis(4,4,4-trifluorobutenoate) (DCPDFC).
[0086] The steps of synthesizing DCPDFC are as follows: First, 0.5 g (the amount of substance is 3.1 mmol) of 2,3-dicyanohydroquinone is dissolved in 50 mL of dry THF, and then excessive 4,4,4-trifluoroquinone is added dropwise. Crotonoyl chloride 2.0 mL. After adding a small amount of triethylamine, the resulting mixture was stirred well for 24 hours. Finally, the solvent was removed by a rotary evaporator, and the product was purified by silica gel column chromatography (ethyl acetate:petroleum ether 1:4). The final product was a white powder with a yield of 36%.
[0087] The reaction formula of 2,3-dicyanohydroquinone and 4,4,4-trifluorocrotonoyl chloride is as follows:
[0088] R-COCl+R'OH→RCOOR'+HCl
[0089] 2. The fluorescence response of 2,3-dicyano-1,4-phenylene bis(4,4,4-trifluorobutenoate) (DCPDFC) to mercapto compounds.
[0090] Add 90 μL of DCPDFC solution at a concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com