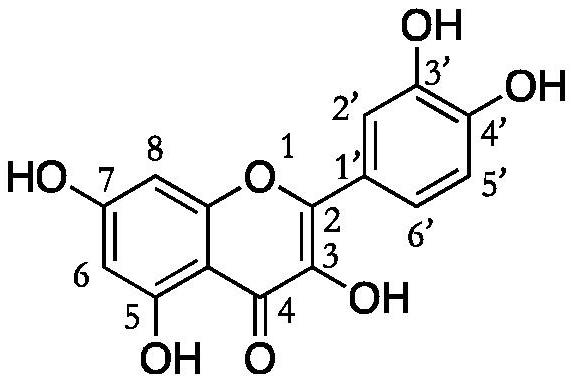
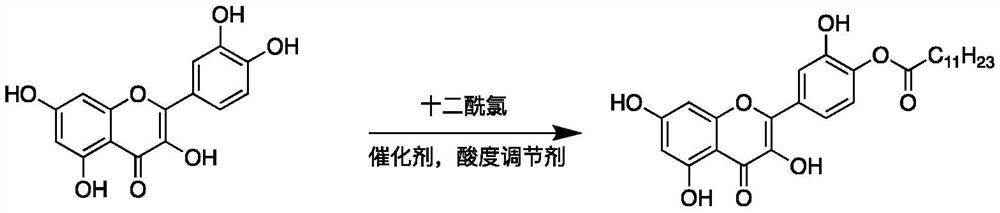
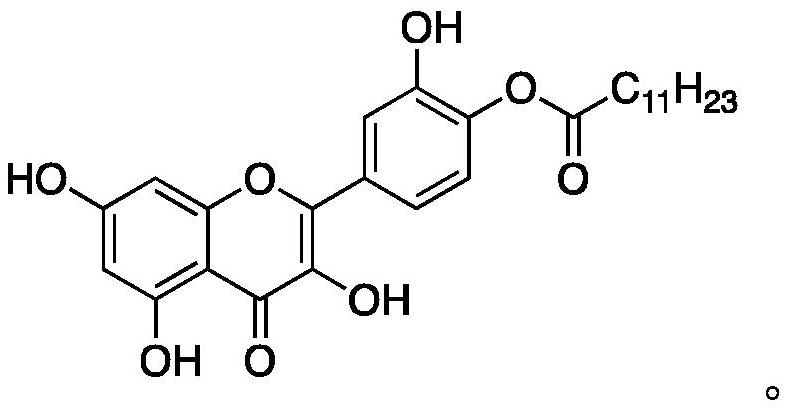
A quercetin derivative chemically modified by dodecanoyl chloride and its synthesis method
A technology of dodecanoyl chloride chemistry and synthesis method, which is applied in the field of quercetin derivatives chemically modified by dodecanoyl chloride and their synthesis, can solve the problems of low bioavailability, poor water solubility of quercetin and the like, and achieves bioavailability The effect of improving, the synthesis method is simple and easy, and the product yield is high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A synthetic method for quercetin derivatives, the synthetic route is to use quercetin and lauryl chloride to directly react the target compound under the action of a catalyst, and the method specifically includes the following steps:
[0033] S1. Add 10 mL of anhydrous DMSO to a 100 mL three-neck flask, then add 151 mg of quercetin, dissolve 163.5 mg of lauryl chloride in 3 mL of anhydrous DMSO, slowly drop into the reaction solution, add 18.6 mg of potassium iodide and 25 mg of potassium carbonate; Use a diaphragm vacuum pump to evacuate, nitrogen protection throughout the process, ice-water bath to room temperature and light-shielding reaction, the speed is 650rpm / min, and the reaction is 10h;
[0034] S2. After the reaction, add 0.1mol / L HCL, adjust the pH value to 6-7, add 30mL ethyl acetate and 10ml pure water, extract, separate the liquid, and continue to extract the water layer twice with 30mL ethyl acetate;
[0035] S3. After combining the organic layers obtaine...
Embodiment 2
[0038] A synthetic method for quercetin derivatives, the synthetic route is to use quercetin and lauryl chloride to directly react the target compound under the action of a catalyst, and the method specifically includes the following steps:
[0039] S1. Add 10 mL of anhydrous DMF to a 100 mL three-necked flask, then add 151 mg of quercetin, dissolve 272.5 mg of dodecanoyl chloride in 10 mL of anhydrous DMF, add 25 mg of potassium carbonate and 15.18 mg of triethylamine, and vacuumize with a diaphragm vacuum pump. Nitrogen protection throughout the process, ice-water bath to room temperature shading reaction, rotation speed 650rpm / min, reaction 10h;
[0040] S2. After the reaction, add 0.1mol / L HCL, adjust the pH value to 6-7, add 30mL ethyl acetate and 10ml pure water, extract, separate the liquid, and continue to extract the water layer twice with 30mL ethyl acetate;
[0041] S3. After merging the organic layers obtained each time, add anhydrous sodium sulfate to it and leave...
Embodiment 3
[0044] A synthetic method for quercetin derivatives, the synthetic route is to use quercetin and lauryl chloride to directly react the target compound under the action of a catalyst, and the method specifically includes the following steps:
[0045] S1. Add 10 mL of anhydrous THF to a 100 mL three-neck flask, then add 151 mg of quercetin, dissolve 136.25 mg of lauryl chloride in 10 mL of anhydrous THF, slowly drop into the reaction solution, add 25 mg of sodium carbonate and 15.18 mg of triethyl For amine, use a diaphragm vacuum pump to evacuate, nitrogen protection throughout the process, ice-water bath to room temperature and light-shielding reaction, the speed is 650rpm / min, and the reaction is 10h;
[0046] S2. After the reaction, add 0.1mol / L HCL, adjust the pH value to 6-7, add 30mL ethyl acetate and 10ml pure water, extract, separate the liquid, and continue to extract the water layer twice with 30mL ethyl acetate;
[0047] S3. After merging the organic layers obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com