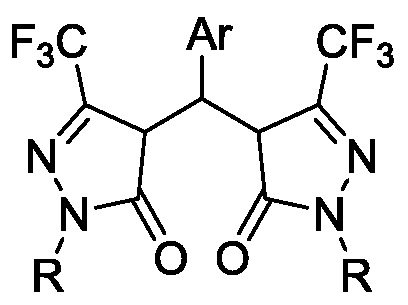
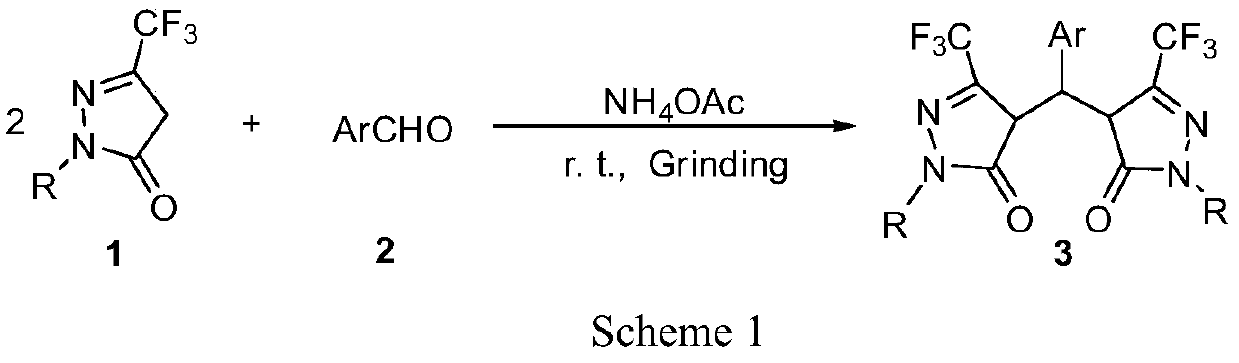
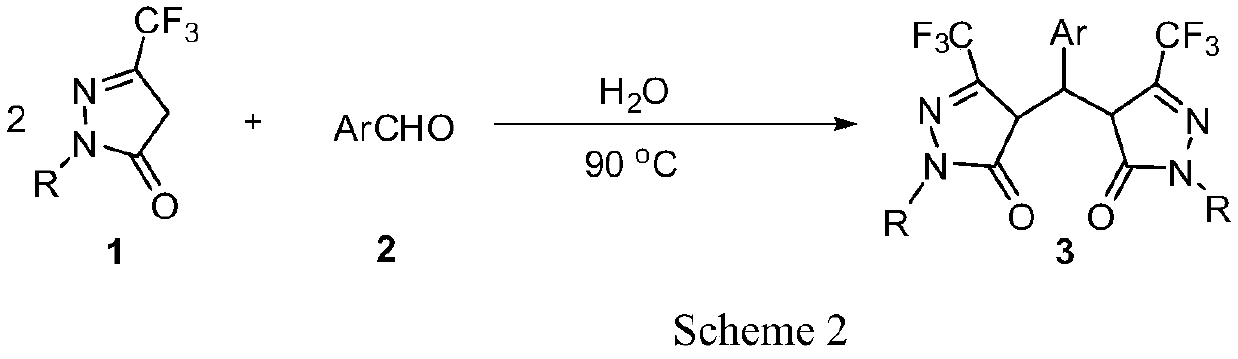
Preparation method of pyrazolone derivative
A pyrazolone and derivative technology, applied in the field of heterocyclic compounds in organic chemistry, can solve the problems of not promoting the best conversion of raw materials, not very environmentally friendly, increasing energy consumption, etc., and achieving excellent yield and reaction time. Short, safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: 1-phenyl-3-trifluoromethyl-5-pyrazolone (456.0mg, 2.0mmol) and benzaldehyde (106.2mg, 1.0mmol) were added to a 25mL round bottom flask, and 5mL ethyl acetate Dissolve the ester, add 7g of crude silica gel, mix well and spin dry with a rotary evaporator. Put a magnet and stir at room temperature under the action of a magnetic stirrer for 12 minutes, take a small amount of the mixture and dissolve it in a solvent, track it on a TLC plate, after the reaction is completed, wash the mixture with an appropriate amount of ethyl acetate, filter to remove the crude silica gel, and remove the filtrate under reduced pressure The solvent was used to obtain the crude product, and the crude product was purified by ethanol recrystallization to obtain the pure product compound 4,4'-(aryl methylene)bis(1-aryl-3-trifluoromethyl-1H-pyrazole-5(4H) -ketone) (490.0 mg, 90%).
[0031] The structure of this compound is:
[0032]
[0033] Molecular formula: C 27 h 19 f 6 N 4...
Embodiment 2
[0044] Example 2: Add 1-phenyl-3-trifluoromethyl-5-pyrazolone (456.0mg, 2.0mmol), 4-nitrobenzaldehyde (152.2mg, 1.0mmol) into a 25mL round bottom flask, Dissolve in 5 mL of ethyl acetate, add 5 g of crude silica gel, mix well and spin dry with a rotary evaporator. Put magnets into the mixture obtained by spin-drying the solvent, stir at room temperature under the action of a magnetic stirrer for 10 minutes, take a small amount of the mixture and dissolve it in a solvent, track it on a TLC plate, after the reaction is completed, wash the mixture with an appropriate amount of ethyl acetate, and filter The crude silica gel was removed, and the filtrate was decompressed to remove the solvent to obtain the crude product, which was purified by ethanol recrystallization to obtain the pure product compound 4,4'-(4-nitrobenzylidene)bis(1-phenyl-3-tri Fluoromethyl-1H-pyrazol-5(4H)-one) (552.4 mg, 97%).
[0045] The structure of this compound is:
[0046]
[0047] Molecular formula:...
Embodiment 3
[0058] Example 3: Add 1-phenyl-3-trifluoromethyl-5-pyrazolone (456.0mg, 2.0mmol), 4-methoxybenzaldehyde (163.4mg, 1.2mmol) in a 25ml round bottom flask , dissolved in 5 mL of ethyl acetate, added 10 g of crude silica gel, mixed evenly, and spin-dried the solvent with a rotary evaporator. Put the magneton into the mixture obtained by spin-drying the solvent, stir at room temperature with a magnetic stirrer for 15 minutes, take a small amount of the mixture and dissolve it in a solvent, and track it on a TLC plate. After the reaction is completed, wash the mixture with an appropriate amount of ethyl acetate and filter it out. Crude silica gel, the filtrate was decompressed to remove the solvent to obtain the crude product, the crude product was purified by ethanol recrystallization to obtain the pure product compound 4,4'-(4-p-methoxybenzylidene)bis(1-phenyl-3- Trifluoromethyl-1H-pyrazol-5(4H)-one) (506.8 mg, 89%).
[0059] The structure of this compound is:
[0060]
[006...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com