3-p-*alkene-1-secondary amine type compound and preparing method and weeding application thereof
A compound and secondary amine technology, which is applied in the field of 3-p-*ene-1-secondary amine compounds and their preparation and herbicide applications, can solve the problems of difficult biodegradation and high toxicity of synthetic pesticides, and achieve high safety and low The effect of wide applicability and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
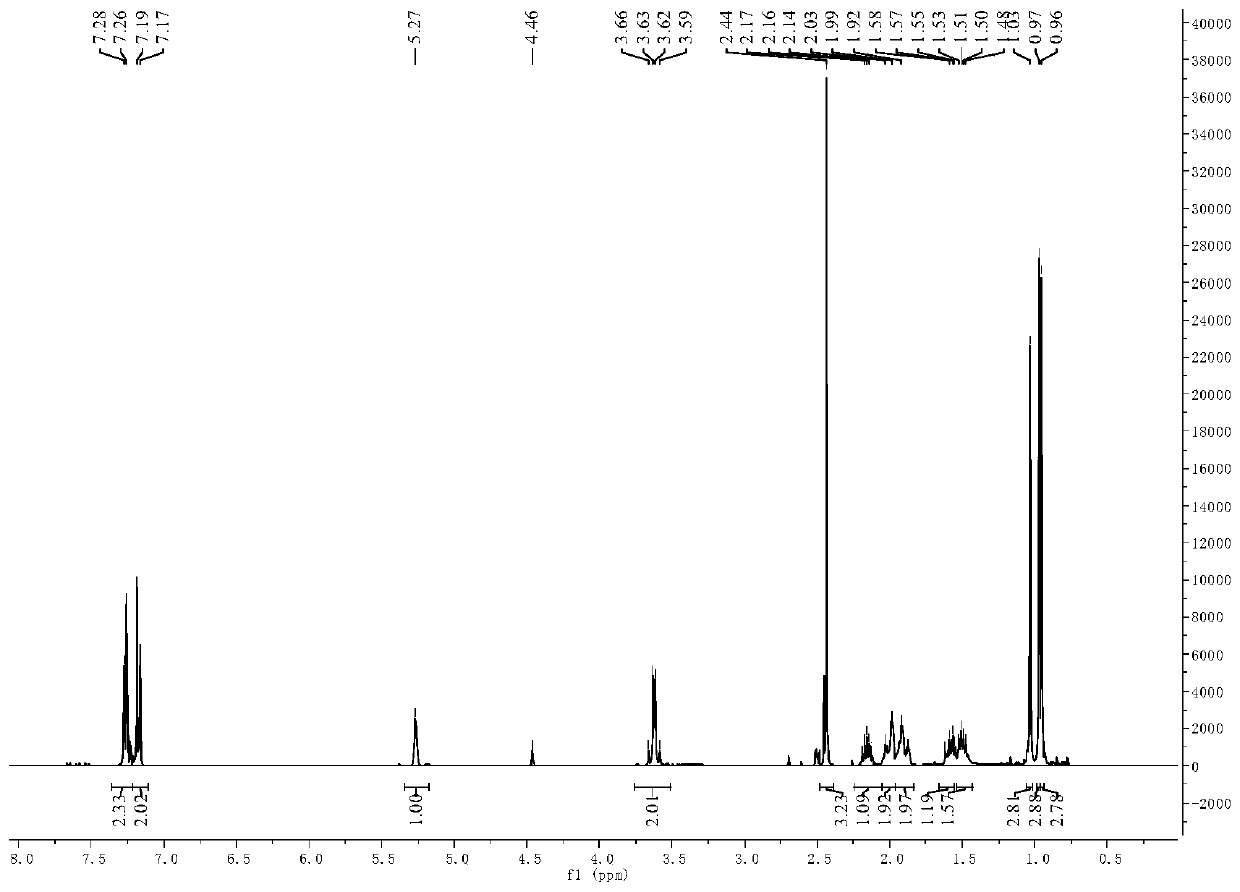
[0052] 5.50g (20mmol) 3-pair En-1-(4-chlorophenyl) Schiff base (self-made. For the preparation method, see: ZL201610947955.7 Examples) was added to a three-necked flask containing 20 mL of methanol, and 1.513 g (40mmol) sodium borohydride, each interval of 30min, after the addition, react at room temperature for 2h, after the reaction is complete, add 20mL of water to quench, extract with dichloromethane, collect the organic phase, wash with saturated brine, and dry over anhydrous sodium sulfate. Filter, remove the solvent under reduced pressure, and then purify by silica gel column chromatography to obtain 3-pair En-1-(4-chlorobenzyl)secondary amine, yield 92%.
[0053] Accurately weigh 1mmol 3-pair En-1-(4-chlorophenyl) Schiff base and 3-p Alkene-1-(4-methylthiobenzyl) secondary amines were dissolved in 0.25mL DMF, and a drop of Tween 80 was added dropwise. After fully dissolved, transferred to a 100mL volumetric flask and diluted to the mark with distilled water to o...
Embodiment 2
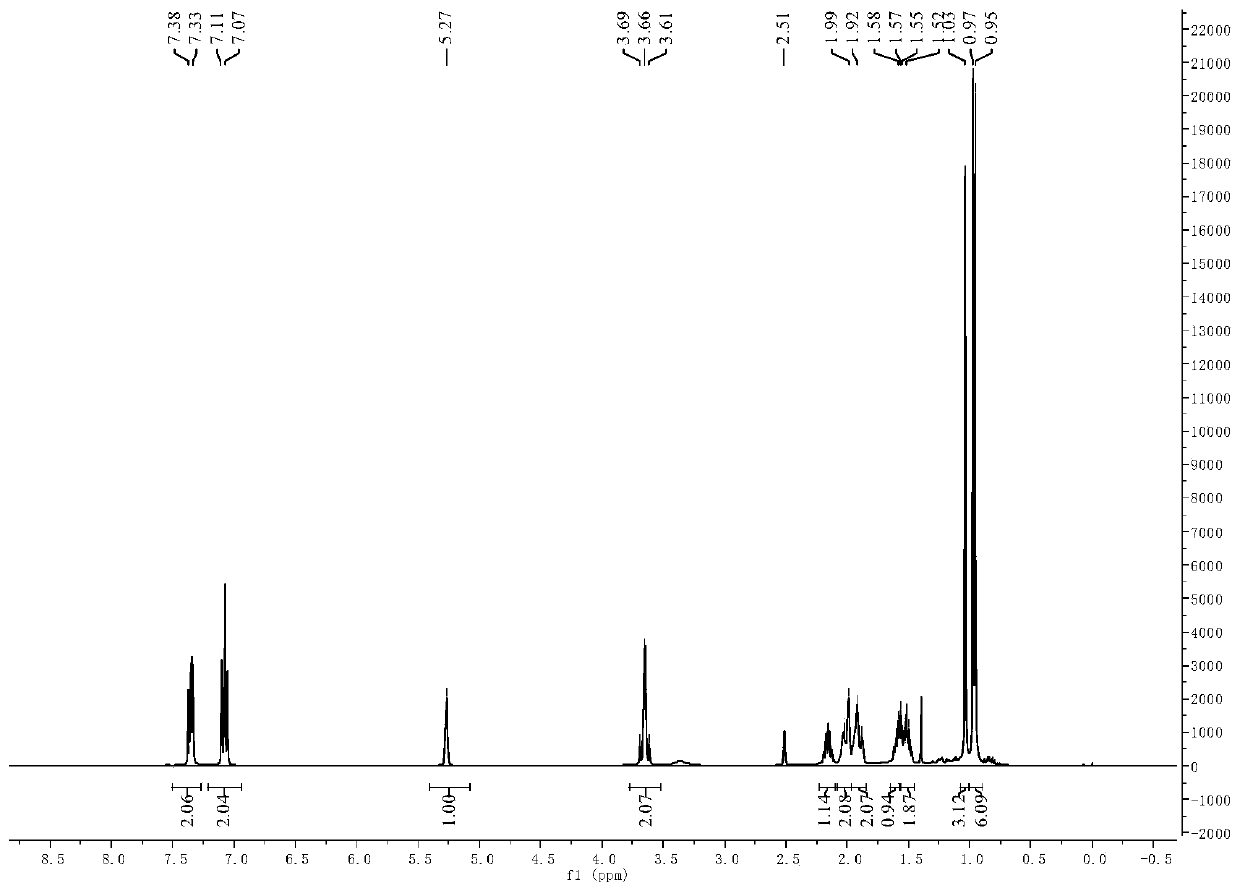
[0056] In addition to raw materials 3-pair En-1-amine Schiff bases are 3-pair Alkene-1-(4-fluorophenyl) Schiff base, other operation process is the same as embodiment 1, target product 3-pair The yield of en-1-(4-fluorobenzyl) secondary amine was 76.0%. 3-pairs of 10mmol / L, 5mmol / L, 2.5mmol / L, 1.25mmol / L, 0.625mmol / L and 0.3125mmol / L The inhibitory rates of alkenyl-1-(4-fluorophenyl) Schiff base solution to barnyardgrass seed root length are respectively: 88.6%, 87.9%, 86.1%, 65.8%, 41.0% and 26.8%, and the inhibition to stem length The rates were: 97.5%, 94.7%, 74.3%, 56.4%, 44.5% and 40.0%. 3-pairs of 10mmol / L, 5mmol / L, 2.5mmol / L, 1.25mmol / L, 0.625mmol / L and 0.3125mmol / L The inhibitory rates of ene-1-(4-fluorobenzyl) secondary amine solution to barnyardgrass seed root length are respectively: 100.0%, 100.0%, 100.0%, 68.0%, 84.2% and 67.0%, and the inhibitory rates to stem length They are: 100.0%, 100.0%, 100.0%, 85.6%, 65.4% and 56.0%.
Embodiment 3
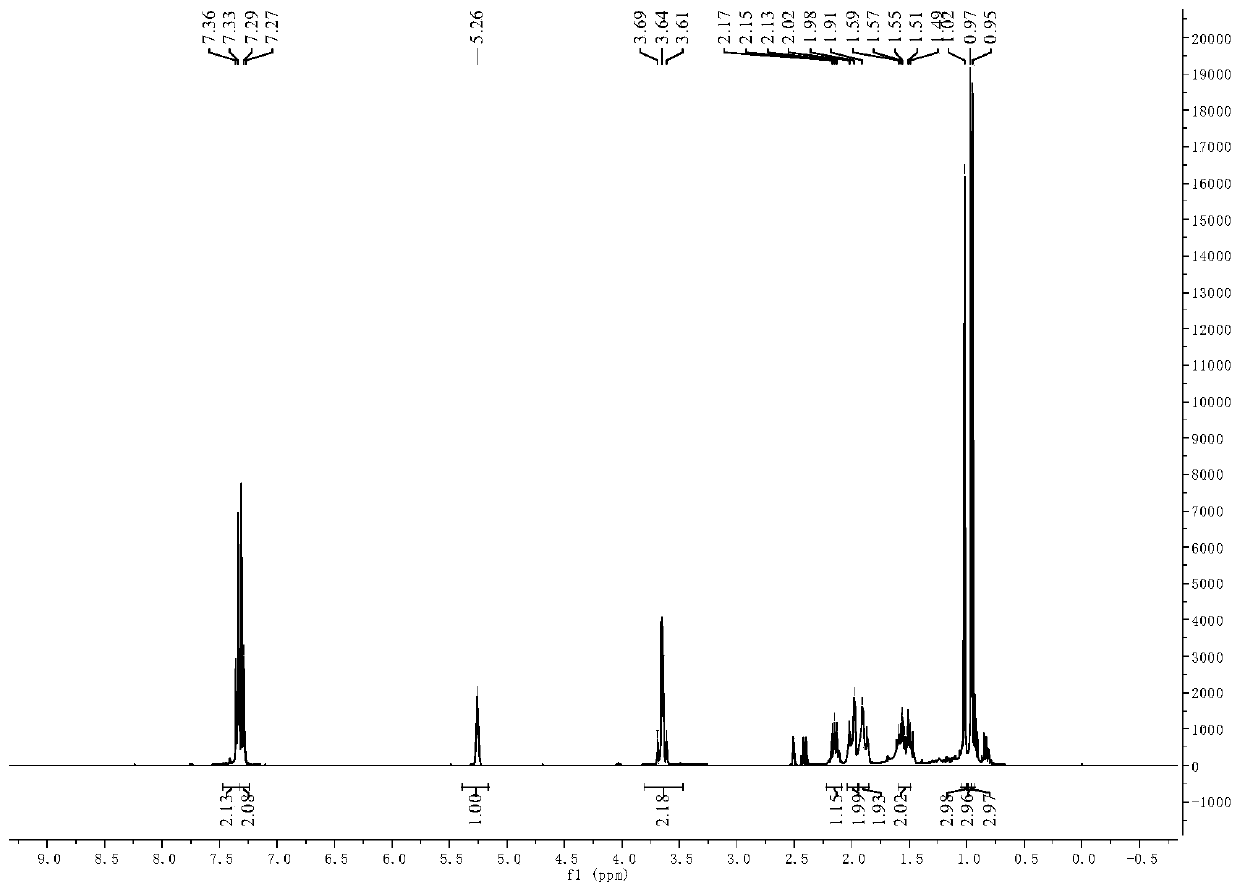
[0058] 3-pair En-1-amine Schiff base derivatives are 3-pair Alkene-1-(4-methylthiophenyl) Schiff base, other operating procedures are the same as in Example 1, and the target product yield is 73%. 3-pairs of 10mmol / L, 5mmol / L, 2.5mmol / L, 1.25mmol / L, 0.625mmol / L and 0.3125mmol / L The inhibitory rates of alkenyl-1-(4-methylthiobenzyl) secondary amine solution to barnyardgrass seed root length were: 71.8%, 71.4%, 69.5%, 64.9%, 63.9% and 53.1%, and the effect on stem length The inhibition rates were: 86.9%, 67.0%, 49.4%, 45.0%, 35.2% and 21.0%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com