Asymmetric terpyridine complex as well as preparation method and application thereof
A terpyridine, asymmetric technology, applied in the direction of copper organic compounds, zinc organic compounds, indium organic compounds, etc., can solve the problems of inability to do on-site detection, time-consuming, high cost, and achieve the effect of double visual recognition and fast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1M is Zn, R is the preparation method of the asymmetric terpyridine complex of ethyl
[0062] step 1:
[0063]
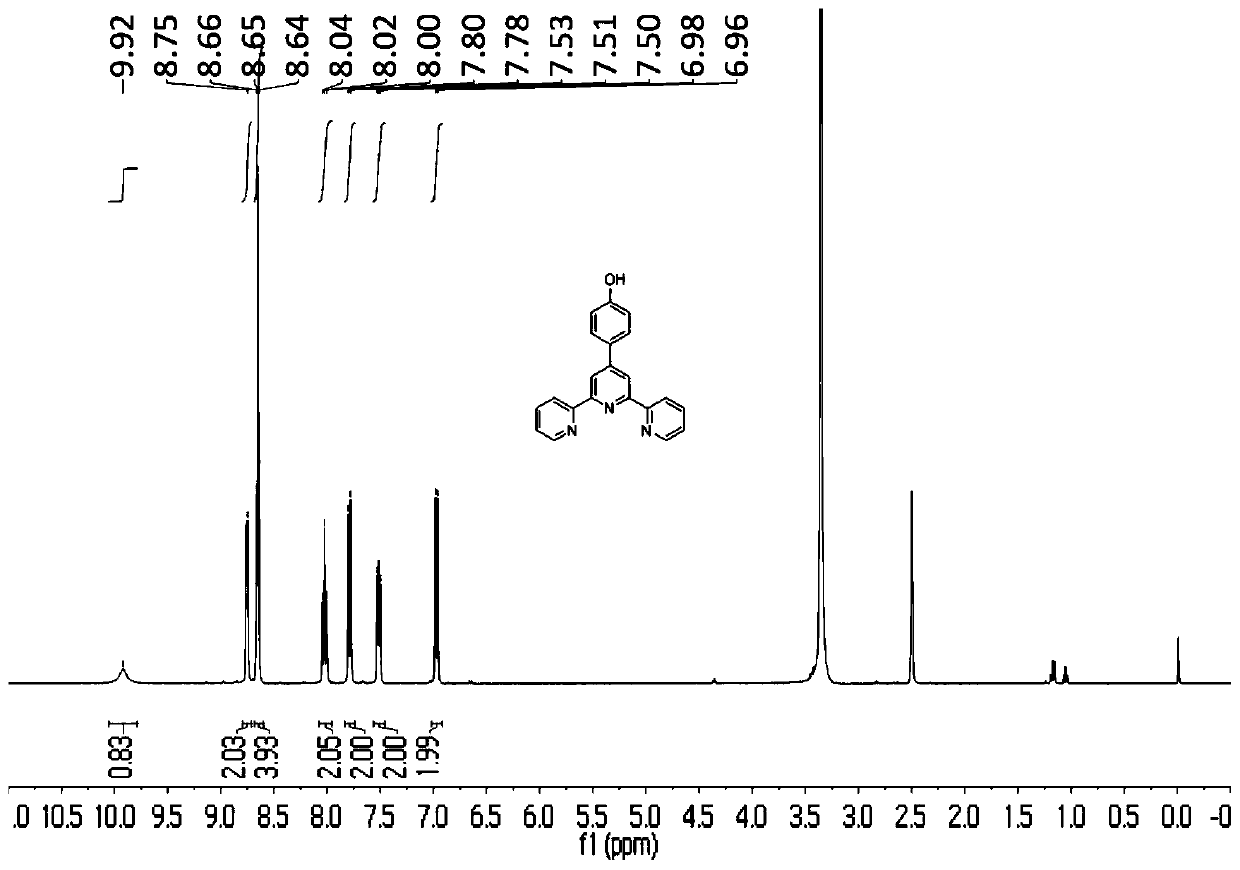
[0064] Add 12.2g of p-hydroxybenzaldehyde and 21g of potassium hydroxide in a 1L round bottom flask, then add 300mL of ethanol, then add 80mL of ammonia water, and finally add 22.4mL of 2-acetylpyridine. 50°C overnight. After the reaction, cool to room temperature, add acetic acid to adjust the pH to 4, the reaction system exotherms a lot and a large amount of yellow solid precipitates out. Suction filter, then wash with 3×30mL ethanol, and dry. Yield 44%. figure 1 Is the proton nuclear magnetic resonance spectrogram of the prepared p-hydroxyphenyl terpyridine. 1 H NMR (400MHz, DMSO-d 6 )δ9.92(s,1H),8.75(s,2H),8.65(t,J=3.9Hz,4H),8.02(t,J=7.7Hz,2H),7.79(d,J=8.5Hz, 2H), 7.56–7.46 (m, 2H), 6.97 (d, J=8.5Hz, 2H).
[0065] Step 2:
[0066]
[0067] Add 6.5 g of p-hydroxyphenyl terpyridine synthesized in step 1 into a 500 mL round bottom fl...
Embodiment 2
[0080] Embodiment 2M is Cu, R is the preparation method of the asymmetric terpyridine complex of ethyl
[0081] The preparation method of step 1 to step 4 of this embodiment is the same as the preparation method of step 1 to step 4 of embodiment 1.
[0082] Step 5:
[0083]
[0084] Add 0.6 g of terpyridine quaternary ammonium salt and 1 mM copper nitrate into a 50 mL round bottom flask, and then add 20 mL of methanol. Stir at room temperature for 5 h, filter to obtain the terpyridine copper complex product, which is washed with methanol and dried. Yield: 73%.
[0085] Step 6:
[0086]
[0087] Add 1mmol terpyridine copper complex and 0.53g terpyridine thymidine into a 50mL round bottom flask, then add 10mL DMF, and react at 80°C for 48h. After the reaction, it was spin-dried and then recrystallized with methanol to obtain an unsymmetrical terpyridine complex (L2). Yield 81%.
Embodiment 3
[0088] Embodiment 3M is Mn, R is the preparation method of the asymmetric terpyridine complex of ethyl
[0089] The preparation method of step 1 to step 4 of this embodiment is the same as the preparation method of step 1 to step 4 of embodiment 1.
[0090] Step 5:
[0091]
[0092] Add 0.6 g of terpyridine quaternary ammonium salt and 1 mM manganese nitrate into a 50 mL round bottom flask, and then add 20 mL of methanol. Stir at room temperature for 5h, filter, and wash the product with methanol and dry it. Yield: 77%.
[0093] Step 6:
[0094]
[0095] Add 1mmol terpyridine manganese complex and 0.53g terpyridine thymidine into a 50mL round bottom flask, then add 10mL DMF, and react at 80°C for 48h. After the reaction, it was spin-dried and then recrystallized with methanol to obtain an unsymmetrical terpyridine complex (L3). Yield: 67%.
[0096] The gel detection melamine of the asymmetric terpyridine complex L1 of embodiment 4 embodiment 1
[0097] Add 10 mg of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com