Drug carrier based on sulfonium lipidosome structure and preparation method and application of drug carrier
A use, ethyl technology, applied in the field of medicine, can solve the problems of strong biological toxicity, poor targeting specificity, low gene expression efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
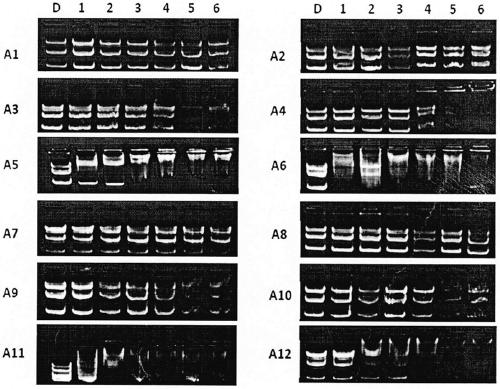
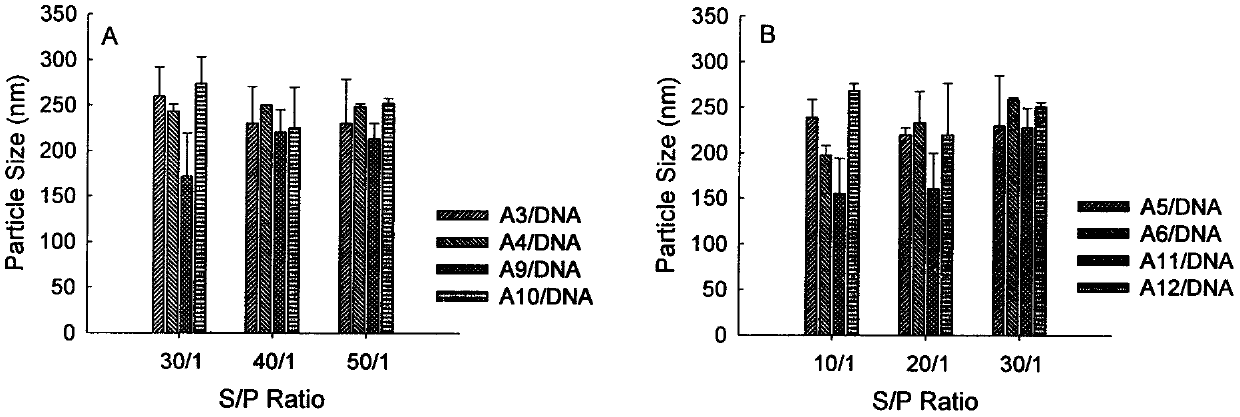
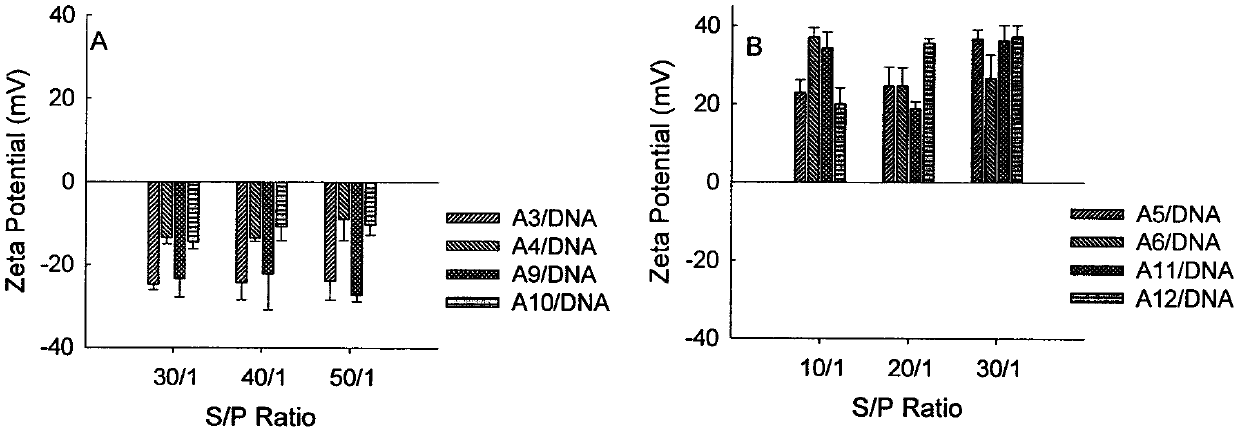
Image
Examples
Embodiment 1
[0053] 2-(2-(2-(Nonyl)ethoxy)ethoxy)ethanol (P1)
[0054] Using the general method of P synthesis, triethylene glycol (2 mL, 14.93 mmol) was reacted with 1-iodononane (3.79 g, 14.93 mmol), sodium hydride (0.90 g, 22.5 mmol) to give P1 (1.93 g, 7.01 mmol, Yield 47%), as colorless oil; R f =0.43 (ethyl acetate-n-hexane 3:2), directly put into the next reaction.
Embodiment 2
[0056] 2-(2-(2-(Dodecyl)ethoxy)ethoxy)ethanol (P2)
[0057] Using the general method of P synthesis, triethylene glycol (2 mL, 14.93 mmol) was reacted with 1-iodododecane (4.42 g, 14.93 mmol), sodium hydride (0.90 g, 22.5 mmol) to give P2 (1.89 g, 5.95 mmol , yield 40%), as a colorless oil; R f =0.41 (ethyl acetate-n-hexane 3:2), directly put into the next reaction.
Embodiment 3
[0059] 2-(2-(2-(Hexadecyl)ethoxy)ethoxy)ethanol (P3)
[0060] Using the general method of P synthesis, triethylene glycol (2 mL, 14.93 mmol) was reacted with 1-iodohexadecane (5.26 g, 14.93 mmol), sodium hydride (0.90 g, 22.5 mmol) to give P3 (1.98 g, 5.52 mmol) , yield 37%), as a colorless oil; R f =0.38 (ethyl acetate-n-hexane 3:2), directly put into the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com