Micromolecule polypeptide and application thereof to preparation of medicines for preventing and treating Parkinson's disease
A technology of medicine and composition, which is applied in the field of application of small molecule peptides in the preparation of drugs for the prevention or treatment of Parkinson's syndrome. Good, non-toxic and side effects, high synthetic purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Artificial synthesis of TAT-pSyn
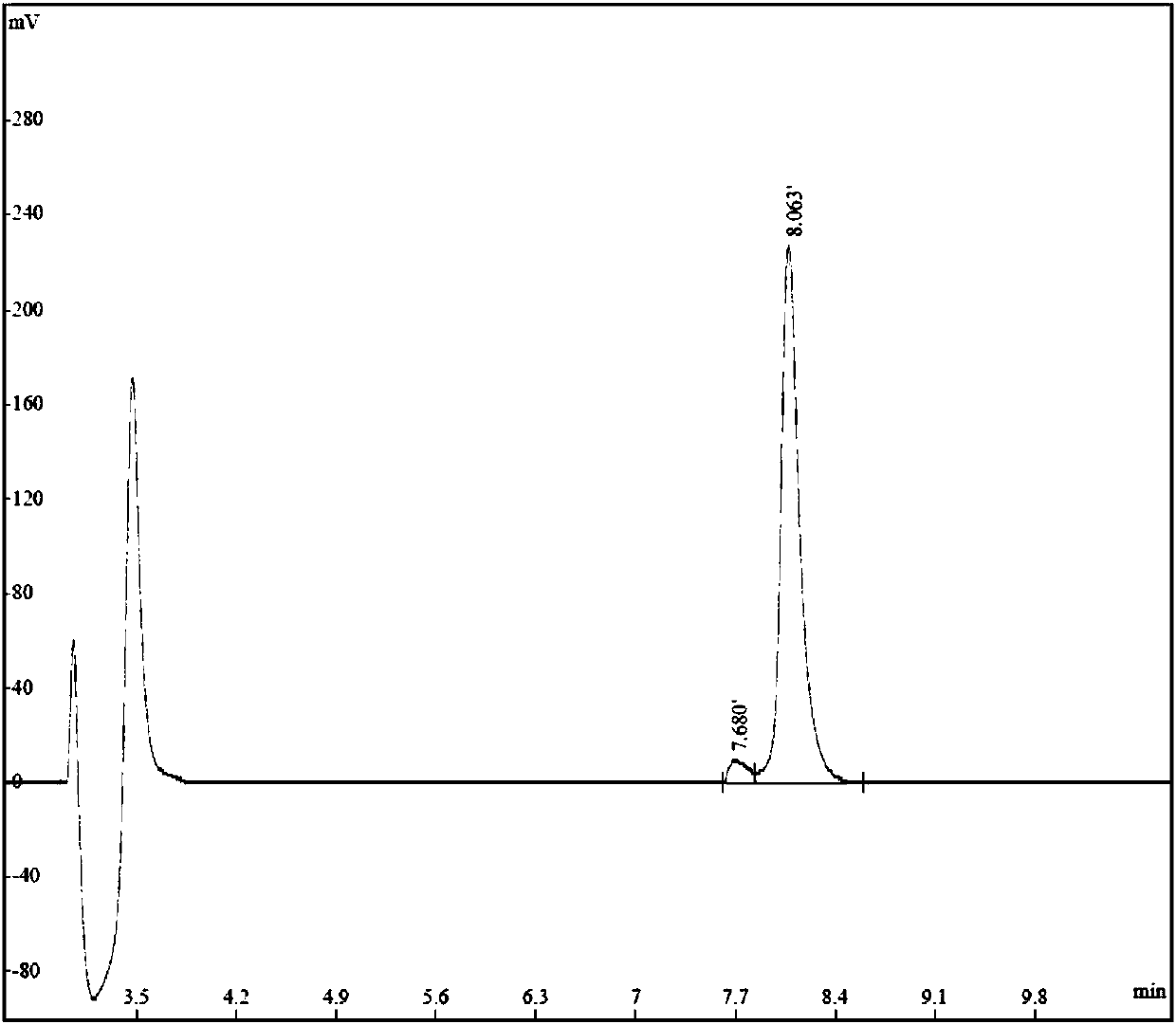
[0032] The sequence of TAT-pSyn is shown in SEQ ID NO.1, artificially synthesized by Jiangsu Qiangyao Biotechnology Co., Ltd., the synthesis report is shown below, and the chromatogram is shown as figure 1 Shown.
[0033] TAT-pSyn synthetic HPLC report
[0034]
[0035] The synthetic TAT-pSyn polypeptide has a purity of 95.79%, 1 mg per tube, is a white powder, completely soluble in water, and stored at -20°C in a sealed and dark place. Before use, dilute it with the injection saline according to the specified concentration and prepare it for immediate use.
[0036] The control of TAT-pSyn is TAT-scramble-pSyn (TAT-s-pSyn), and its sequence is as follows:
[0037] Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Pro-Glu-Met-Ser-Tyr-Gly-Glu-Gln-Tyr-Glu-Asp(YGRKKRRQRRR-PEMSYGEQYED), also Synthesized for the company.
Embodiment 2
[0039] TAT-pSyn blocks the binding of DAPK1 to alpha-synuclein and inhibits the hyperphosphorylation and abnormal aggregation of alpha-synuclein.
[0040] Culture neurofibroma blast cells (N2a) in vitro, transfect the eukaryotic expression alpha-synuclein plasmid, and give 0uM, 5uM, 25uM, 50uM TAT-pSyn polypeptide or control TAT- to the cells co-transfected with DAPK1 plasmid. Incubate with s-pSyn polypeptide or vehicle. After 90 minutes, cell protein was extracted. The results showed that after 25uM TAT-pSyn was given, the protein amount of alpha-synuclein and phosphorylated alpha-synuclein was reduced ( figure 2 ). It suggests that TAT-pSyn blocks the interaction between DAPK1 and alpha-synuclein, thereby inhibiting the hyperphosphorylation and abnormal aggregation of alpha-synuclein.
Embodiment 3
[0042] Application of TAT-pSyn in a transgenic mouse model of Parkinson's syndrome
[0043] (1) Establishment of a mouse model of Parkinson's syndrome
[0044] A transgenic mouse of Alpha-synuclein (A53T) No. 004479 was purchased from Jackson Lab in the United States for breeding. The transgenic mouse expresses human-derived A53T mutant synuclein (alpha-synuclein, full length 140 amino acids). Mouse prion protein promoter. At eight months, some homozygous mice gradually showed severe movement disorders. The phenotype manifests for an average of 14-15 months. Manifestations include sagging skin, weight loss and dyskinesia that precedes dyskinesias, paralysis of some limbs, tremors, and inability to stand. Immunohistochemical analysis of mutant mice aged 8-12 months revealed that synuclein content aggregates in the spinal cord, brainstem, cerebellum, and striatum. The malignant aggregation of synuclein is synchronized with the development of movement disorders. We use 12-month-o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com