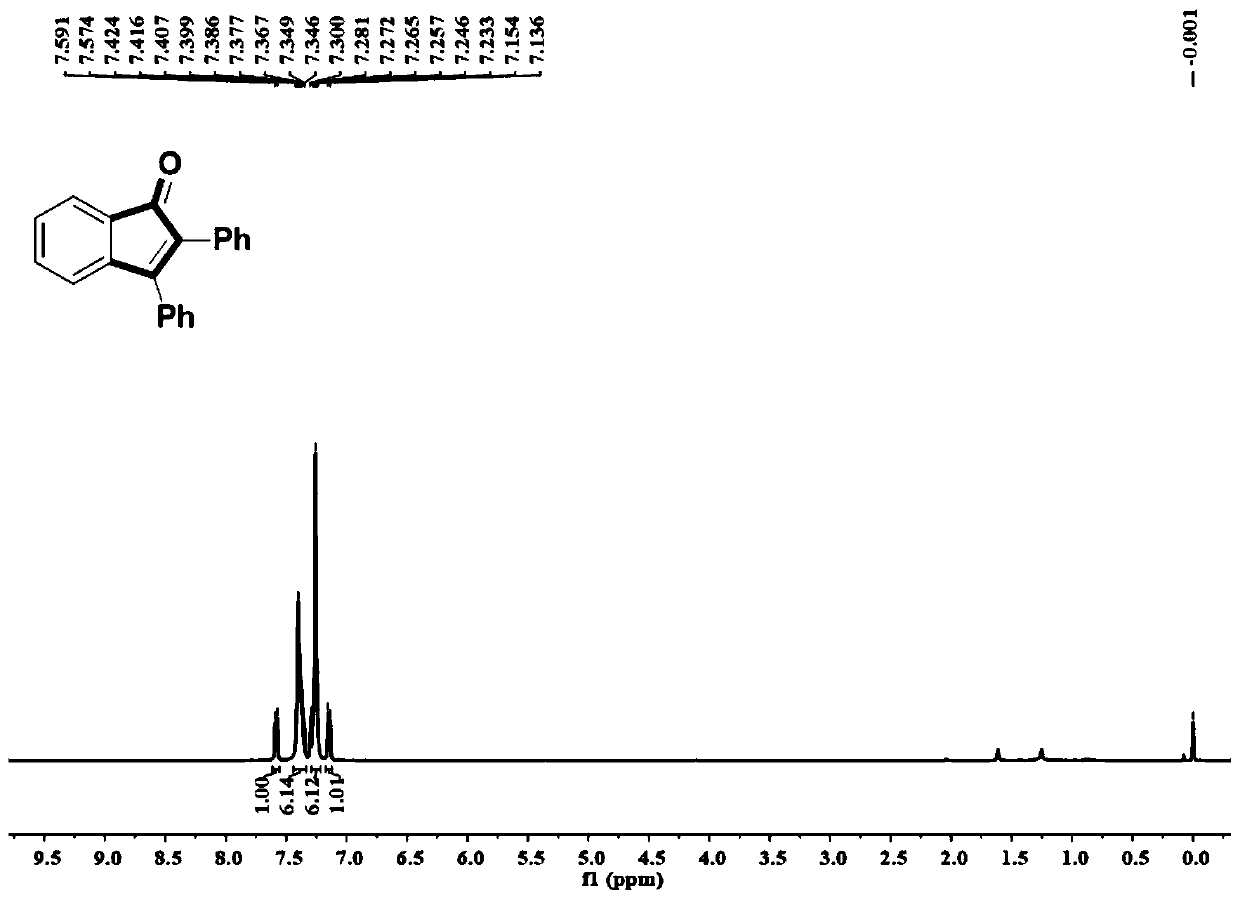
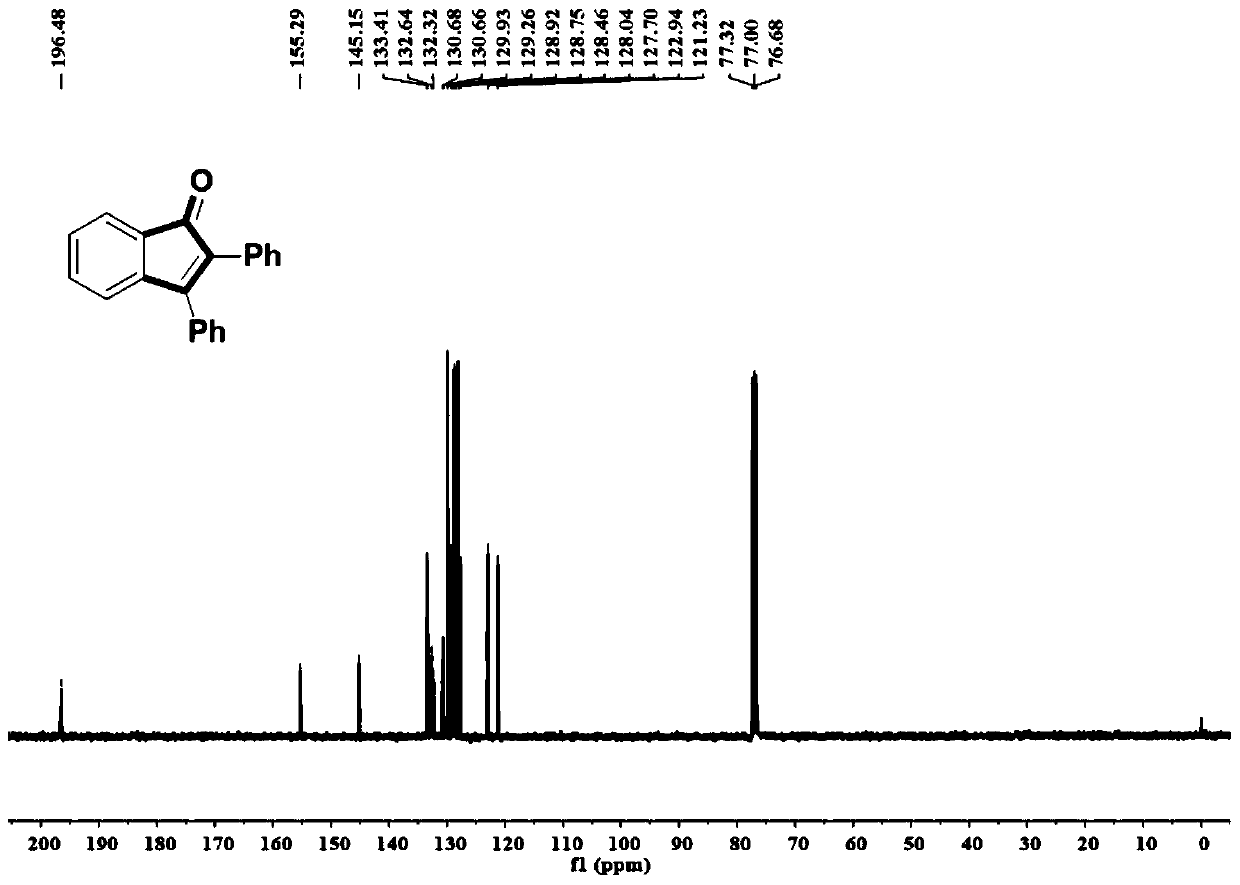
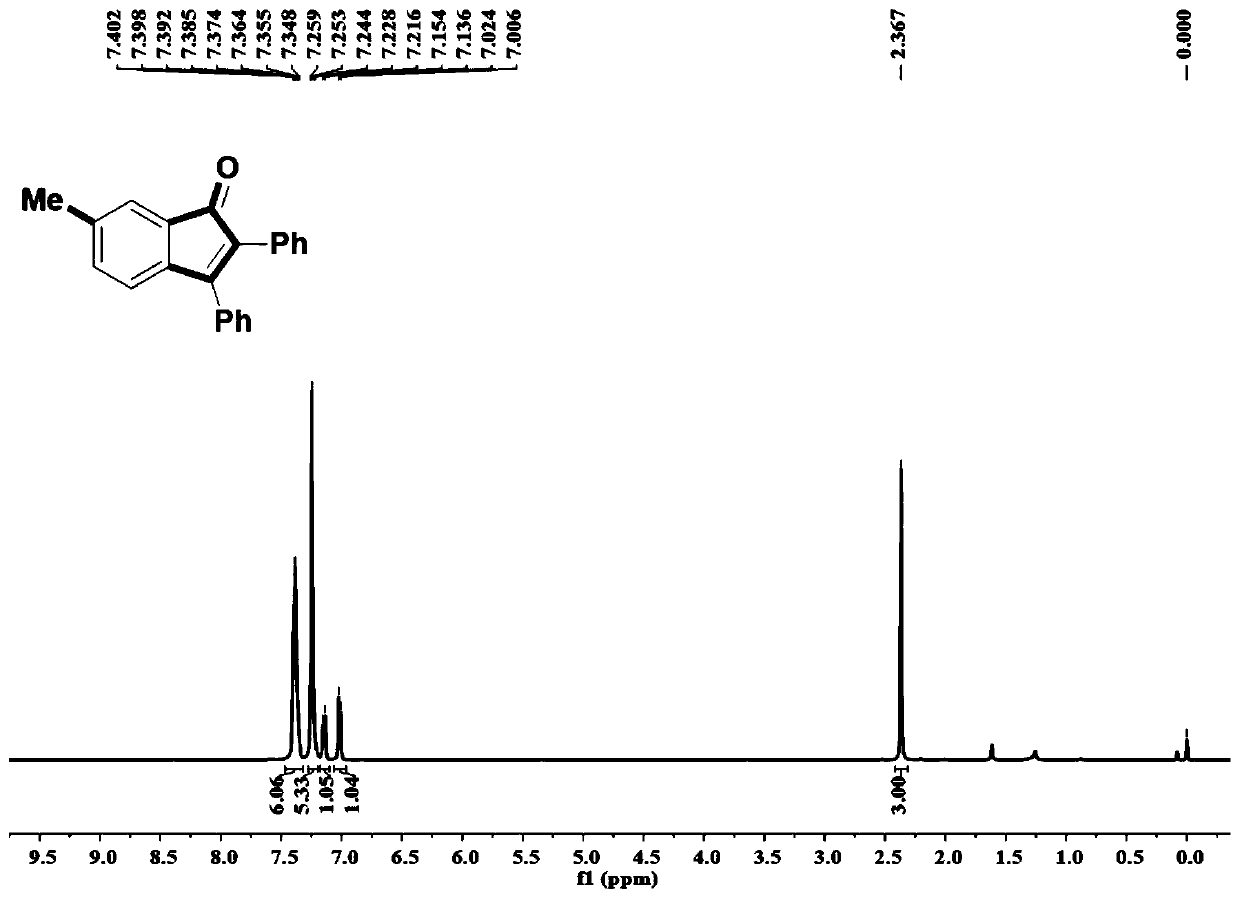
Synthetic method of indanone and derivatives thereof
A synthesis method and derivative technology, applied in carbon monoxide reaction preparation, organic chemistry, etc., can solve the problems of difficult commercial purchase, complex raw materials, and difficulty in large-scale practical application, and achieve good substrate applicability and simple synthesis method , good expansibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] A method for synthesizing indanone and derivatives thereof of the present invention, the steps are: adding dihydrocarbyl acetylene compounds, palladium catalysts, additives and inorganic bases together into a dry reaction vessel, and replacing the gaseous atmosphere in the reaction vessel with air It is carbon monoxide; under a carbon monoxide gas atmosphere at standard atmospheric pressure, add o-bromoiodobenzene compounds and anhydrous 1,4-dioxane solvent, heat to 100°C, react for 24 hours, and then cool to room temperature; add saturated chlorine Ammonium chloride solution quenches the reaction, then adds water, extracts with ethyl acetate, separates by column chromatography, and purifies to obtain indanone and its derivatives. The reaction formula is:
[0055]
[0056] Among them, o-bromoiodobenzene compounds are o-bromoiodobenzene, 3-bromo-4-iodotoluene, 2-bromo-4-fluoro-1-iodobenzene, 2-bromo-4-chloro-1-iodobenzene, 2 -Bromo-1-iodo-4-(trifluoromethoxy)benzene, ...
Embodiment 1
[0059] Add 0.25mmol of toluene, 0.025mmol of palladium chloride, 0.25mmol of tetrabutylammonium bromide and 0.5mmol of sodium carbonate into a dry Schlenk tube, replace the gas atmosphere in the Schlenk tube from air to carbon monoxide, and keep the system at a standard atmospheric pressure The carbon monoxide atmosphere was replaced three times to ensure that the system has a pure carbon monoxide atmosphere; add 0.5mmol o-bromoiodobenzene and 1mL of 1,4-dioxane solvent, react at 100°C for 24 hours, and cool to room temperature; add 4mL Quenched by saturated ammonium chloride solution, extracted by adding 15 mL of ethyl acetate three times, combined the organic phases, dried with anhydrous sodium sulfate, and separated by column chromatography to obtain the product with a yield of 97%.
Embodiment 2-8
[0061] The difference from Example 1 is that the o-bromoiodobenzene compounds added in Examples 2-8 are 3-bromo-4-iodotoluene, 2-bromo-4-fluoro-1-iodobenzene, 2- Bromo-4-chloro-1-iodobenzene, 2-bromo-1-iodo-4-(trifluoromethoxy)benzene, 1-bromo-4-fluoro-2-iodobenzene, 1-bromo-4-chloro -2-iodobenzene, 4-bromo-2-iodo-4-(trifluoromethyl)benzene replaces the o-bromoiodobenzene in example 1, and other preparation steps remain unchanged. The reactant and productive rate of embodiment 1-8 are as table 1.
[0062] The general reaction formula of embodiment 1-8 is:
[0063]
[0064] Table 1 Reaction of different o-bromoiodobenzenes with tolan
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com