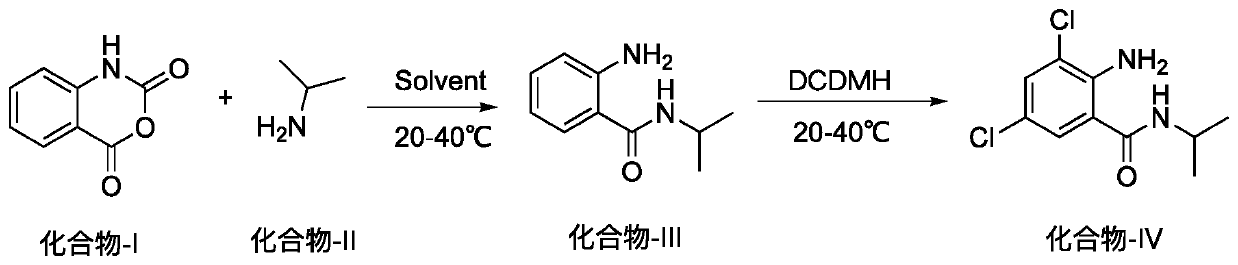
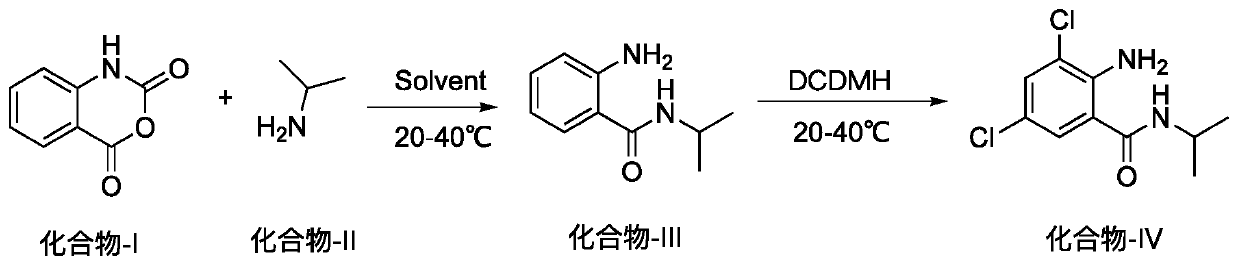
Method for preparing 2-amino-3,5-dichloro-N-isopropylbenzamide
A technology of isopropyl benzamide and isopropylamine, which is applied in the field of preparing 2-amino-3, can solve the problems of large environmental pollution, extraordinary production requirements, and high cost of three wastes treatment, and achieve high total yield and simple post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] At room temperature, in a 1000ml round bottom flask, add isatoic anhydride (50g, 306.5mmol), dichloroethane 300ml, and slowly add isopropylamine (20.8g, 352.4mmol) dropwise at 30-35°C. After the dropwise addition, the reaction was completed after stirring for about 2 hours, and the liquid phase detection of isatoic anhydride was less than 2%. Cool down to 20°C, add dichlorhein (66.4g, 337.2mmol) in batches, keep the temperature at 20-25°C, stir for 12h, the liquid phase detection of compound III is less than 1%, stop the reaction. When about 150ml of the reaction system was distilled off under reduced pressure, the temperature of the system was lowered to below 5°C, filtered with suction, and the solid was washed twice with 50°C water (100ml) and dried by suction. Transfer to a reaction bottle, add anhydrous methanol (200ml), heat to 60°C, cool to below 5°C, filter with suction, and dry to obtain 62g of white solid, the yield of 2 steps is 83%, the purity of liquid phas...
Embodiment 2
[0017] At room temperature, add isatoic anhydride (3kg, 18.39mol) and 18L dichloroethane into a 50L reactor, and slowly add isopropylamine (1.248kg, 21.144mol) dropwise at 30-35°C. After the dropwise addition, the reaction was completed after stirring for about 2 hours, and the liquid phase detection of isatoic anhydride was less than 2%. Cool down to 20°C, add dichlorhein (3.984kg, 20.232mol) in batches, keep the temperature at 20-25°C, stir for 13h, the liquid phase detection of compound III is less than 1%, stop the reaction. When about 10 L of the reaction system was distilled off under reduced pressure, the temperature of the system was lowered to below 5°C, filtered with suction, and the solid was washed twice with 50°C water (2L) and dried by suction. Transfer to a reaction kettle, add anhydrous methanol (10L), heat to 60°C, cool to below 5°C, filter with suction, and dry to obtain 3.68kg of white solid, the yield of 2 steps is 81%, the purity of liquid phase detection ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com