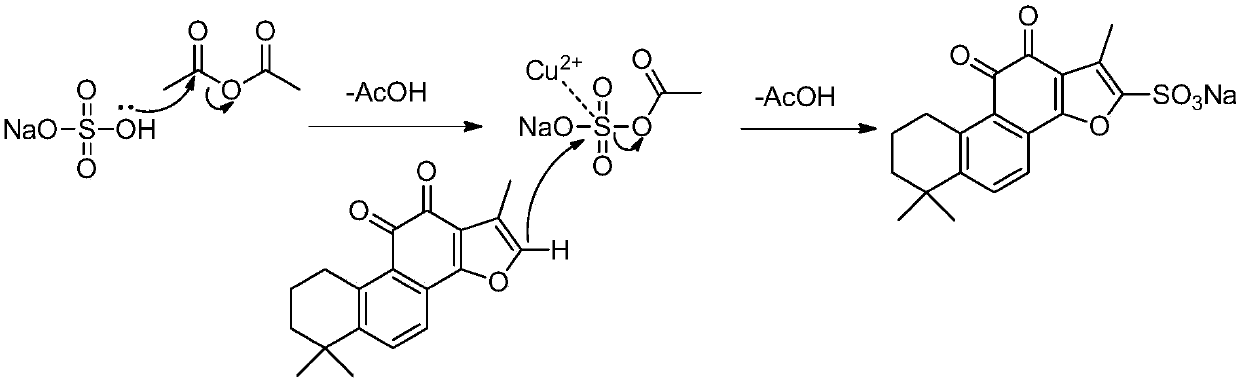
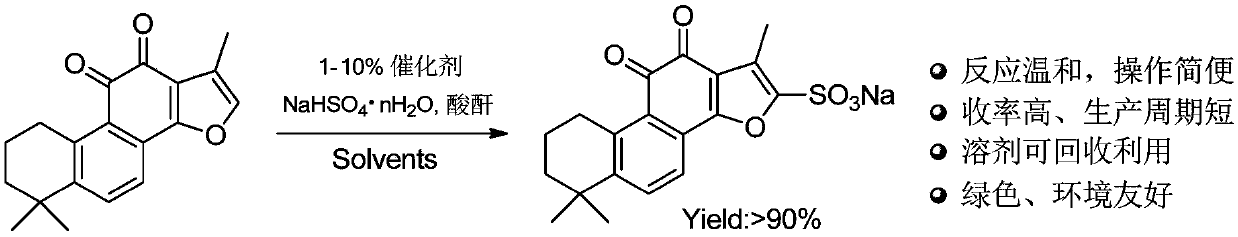
Preparation method for green synthesis of tanshinone IIA sodium sulfonate
A technology of green synthesis and tanshinone, which is applied in the field of medicine, can solve the problems of harsh reaction conditions, reagent stability, poor safety, and difficult recovery of pyridine sulfur trioxide, and achieve safe and controllable reaction process, safe reaction reagents, and short production cycle. shortened effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Cu(OAc) 2 Catalytic Synthesis and Preparation of Sodium Tanshinone IIA Sulfonate
[0048] Tanshinone IIA (2.94 g, 10 mmol) was dissolved in 50 ml of acetic anhydride solution containing sodium bisulfate (1.44 g, 12 mmol); then 10 mol% of the catalyst Cu(OAc) was added 2 (0.18 g, 1 mmol), and at 60 o C is heated until the reaction is complete. After the TLC detection reaction was completed, the anhydride solution of the reaction system was recovered under reduced pressure, and the remaining red solid was added to 100 ml ethanol solution, and the insoluble matter was filtered off; Standing and crystallizing overnight; filtering to obtain 3.67 g of tanshinone IIA sodium sulfonate crystals, with a yield of 92.7% and a content of 99%. Correlation spectrum data of sodium tanshinone IIA sulfonate: HRMS (ESI): [M+Na] + m / z: 396.0647. UV (MeOH) λmax (nm): 202, 226, 225, 271, 279. IR (KBr) (cm -1 ): 3000~2840, 1695, 1674, 1576, 1539, 1459, 1245, 1217, 1041, 923,...
Embodiment 2
[0049] Example 2 Synthesis and preparation of Tanshinone IIA sodium sulfonate catalyzed by aza-15-crown ether-5
[0050] Tanshinone IIA (2.94 g, 10 mmol) was dissolved in 30 ml of acetic anhydride solution containing sodium bisulfate (1.44 g, 12 mmol); then 10 mol% of the catalyst aza-15-crown-5 (0.11 g, 0.5 mmol), and reacted at room temperature for 1 hour to complete. After the TLC detection reaction was completed, dichloromethane was added until a large amount of red solids were precipitated, allowed to cool and then filtered, and the gained red solids were added to 100 ml of ethanol-methanol mixed solution (V 乙醇 :V 甲醇 = 4:1), filtered to remove insoluble matter; the obtained filtrate was concentrated until a small amount of solid precipitated, left to stand, and continued to crystallize overnight at room temperature; filtered to obtain 3.80 g of tanshinone IIA sodium sulfonate crystals, with a yield of 96%. The content is 98%. Related spectral data of sodium tanshinone ...
Embodiment 3
[0051] Example 3 Recovery of acetic anhydride for the synthesis and preparation of tanshinone IIA sodium sulfonate
[0052] The dichloromethane / acetic anhydride filtrate used in the reaction of Example 2 was concentrated under reduced pressure to recover dichloromethane, and then Tanshinone IIA (2.94 g, 10 mmol) and sodium bisulfate (1.44 g, 12 mmol) and 10 mol% catalyst Cu(OAc) 2 (0.18 g, 1 mmol), and at 80 o C is heated until the reaction is complete. After the reaction was detected by TLC, according to the post-treatment method of Example 2, 3.60 g of tanshinone IIA sodium sulfonate crystals were obtained, with a yield of 91% and a content of 99%. Related spectral data of sodium tanshinone IIA sulfonate: 1 H NMR (500MHz, DMSO- d 6 , TMS) δ ppm : 7.85 (1H, d, J = 8.4 Hz,H-6), 7.57 (1H, d, J = 8.4 Hz, H-7), 3.08 (2H, t, J = 6.1 Hz, H-1), 2.33 (3H, s, 17-CH 3 ), 1.72 (2H, m, H-2), 1.62 (2H, m, H-3), 1.29 (6H, s, 18,19-CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com