Preparation method and application of (4-ferroceneethynyl)aniline modified fullerene
A technology of ferroceneacetylene and fullerene, which is applied in the field of solar cells, can solve the problems of low photoelectric conversion efficiency of dye-sensitized solar cells, easy desorption of dyes, and low conductivity, so as to overcome low conductivity and enhance The effect of improving electrical conductivity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
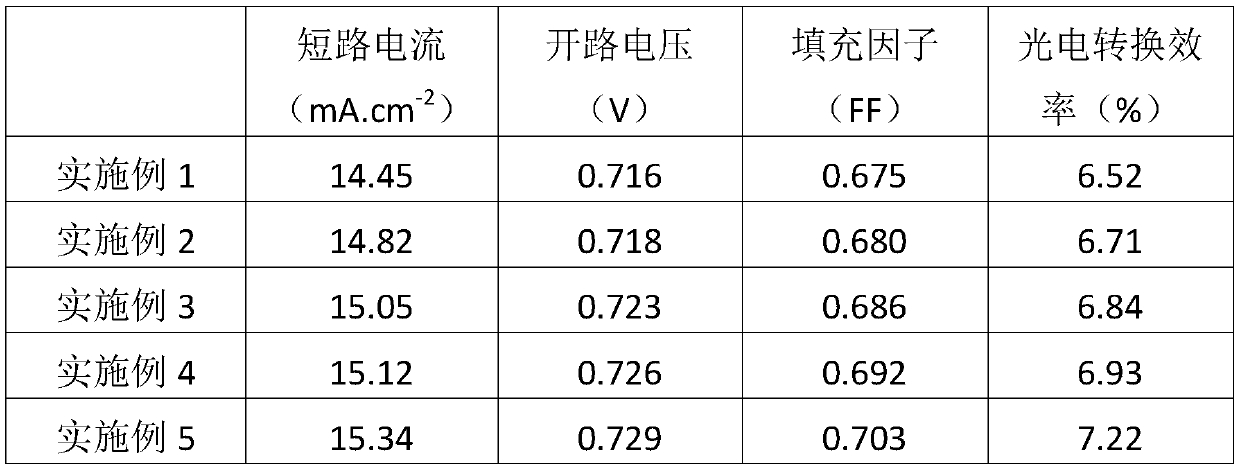
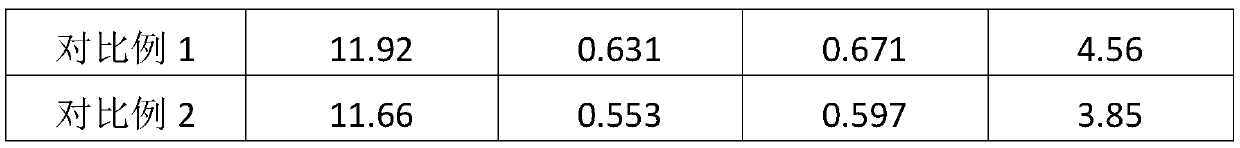
Examples
Embodiment 1
[0031] A solid electrolyte for dye-sensitized solar cells based on ionic crystals, comprising the following components by weight: 40 parts of cyanoimidazole-type ionic crystals, 3 parts of (4-ferroceneethynyl) aniline modified fullerenes, 5 parts of simple iodine, 5 parts of methylbenzimidazole (MBI).
[0032] The preparation method of described cyanoimidazole type ion crystal comprises the steps:
[0033] 1) Preparation of 1-methyl-4,5-dicyanoimidazole: Dissolve 10 g of 4,5-dicyanoimidazole in 100 g of acetonitrile, and then add 15 g of methyl iodide and 5 g of sodium hydroxide to it, at 40 ° C Stirring and reacting for 8 hours, after filtering, adding water to the reaction system, separating the liquid to take the organic phase, and rotary evaporating to remove the organic solvent, to obtain 1-methyl-4,5-dicyanoimidazole;
[0034] 2) Preparation of cyanoimidazole-type ionic crystals: 15 g of 1-methyl-4,5-dicyanoimidazole prepared in step 1) was dissolved in 60 g of ether, a...
Embodiment 2
[0040]A solid electrolyte for dye-sensitized solar cells based on ionic crystals, comprising the following components by weight: 43 parts of cyanoimidazole-type ionic crystals, 4 parts of (4-ferroceneethynyl) aniline modified fullerenes, 7 parts of simple iodine, 6 parts of butylbenzimidazole (NBB).
[0041] The preparation method of described cyanoimidazole type ion crystal comprises the steps:
[0042] 1) Preparation of 1-ethyl-4,5-dicyanoimidazole: 10 g of 4,5-dicyanoimidazole was dissolved in 120 g of chloroform, and 17 g of ethyl iodide and 6 g of potassium hydroxide were added thereto, at 45 Stir the reaction at ℃ for 8.5 hours, then filter, add water to the reaction system, separate the liquid to take the organic phase, and remove the organic solvent by rotary evaporation to obtain 1-ethyl-4,5-dicyanoimidazole;
[0043] 2) Preparation of cyanoimidazole-type ionic crystals: 17 g of 1-ethyl-4,5-dicyanoimidazole prepared in step 1) was dissolved in 78 g of ether, and ethy...
Embodiment 3
[0049] A solid-state electrolyte for dye-sensitized solar cells based on ionic crystals, comprising the following components by weight: 46 parts of cyanoimidazole-type ionic crystals, 5 parts of (4-ferroceneethynyl) aniline-modified fullerenes, 7 parts of simple iodine, 7 parts of tert-butylpyridine (TBP).
[0050] The preparation method of described cyanoimidazole type ion crystal comprises the steps:
[0051] 1) Preparation of 1-propyl-4,5-dicyanoimidazole: 10 g of 4,5-dicyanoimidazole was dissolved in 135 g of acetonitrile, and then 18 g of 1-iodopropane and 7.5 g of sodium carbonate were added thereto. Stir and react at 50°C for 9 hours, then filter, add water to the reaction system, separate the liquid to take the organic phase, and remove the organic solvent by rotary evaporation to obtain 1-propyl-4,5-dicyanoimidazole;
[0052] 2) Preparation of cyanoimidazole-type ionic crystals: 18 g of 1-propyl-4,5-dicyanoimidazole prepared in step 1) was dissolved in 80 g of ether,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com