Chimeric antigen receptor-modified T cells capable of self-expressing PD-1 antibody and targeting mesothelin and uses thereof
A chimeric antigen receptor, PD-1 technology, applied in the fields of genetic engineering and immunology, can solve the problems of high production cost, low response rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
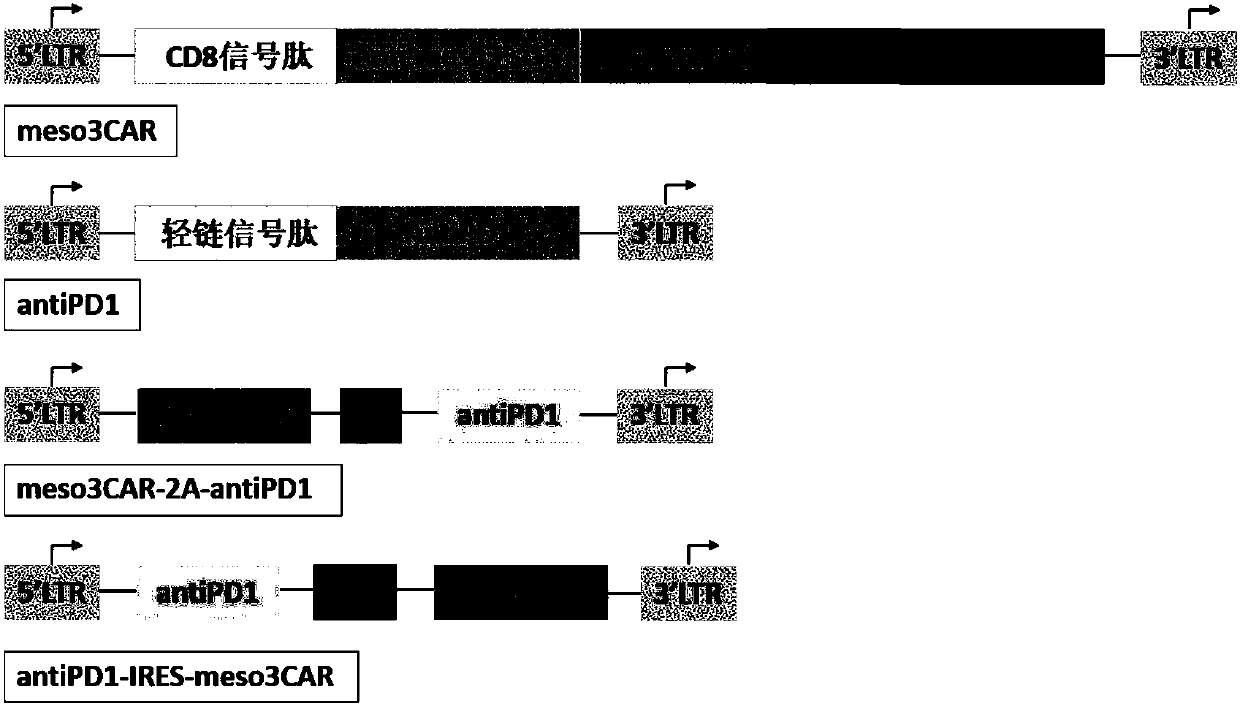
[0101]Example 1: Construction of recombinant plasmids pNB328-meso3CAR, pS328-antiPD1, pNB328-meso3CAR-2A-antiPD1 and pNS328-antiPD1-IRES-meso3CAR
[0102] Entrust commercial companies to artificially synthesize the coding sequence of meso3CAR (SEQ ID NO: 3), the coding sequence of antiPD1 (SEQ ID NO: 4), the coding sequence of meso3CAR-2A-antiPD1 (the coding sequence of 2A is shown in SEQ ID NO: 5) The amino acid sequence is shown in SEQ ID NO: 6) and the coding sequence of antiPD1-IRES-meso3CAR (the coding sequence of IRES is shown in SEQ ID NO: 7), and its structural pattern is shown in figure 1 shown. Each sequence was loaded between the EcoRI and SalI restriction sites of pNB328 and pS328 vectors (see CN 201510638974.7 for the structure and sequence of pNB328, the entire contents of which are incorporated herein by reference; compared with pNB328, pS328 lacks PB transduction transposon, other elements are the same as pNB328), and the constructed recombinant plasmids were ...
Embodiment 2
[0103] Example 2: Construction of mesothelin-targeted CAR T cells expressing PD1 antibody
[0104] Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll separation. Cultivate PBMCs for 2-4 hours, and the unattached suspension cells are initial T cells. Collect the suspension cells into a 15ml centrifuge tube, centrifuge at 1200rmp for 3min, discard the supernatant, add physiological saline, centrifuge at 1200rmp for 3min, discard the physiological saline, and repeat this step; take four 1.5ml centrifuge tubes and add 5×10 6 Cells, numbered a, b, c, centrifuged at 1200rmp for 3min, discarded the supernatant, took the electrotransfer kit (from Lonza company), a, b, c tubes were added in proportion to a total of 100ul of electrotransfer reagents, a tube was added to the constructed recombinant plasmid 4ug each of pNB328-meso3CAR and pS328-antiPD1, add 6ug of pNB328-meso3CAR-2A-antiPD1 plasmid to tube b, add 6ug of pNB328-antiPD1-IRES-meso3CAR plasmid to tube c; Se...
Embodiment 3
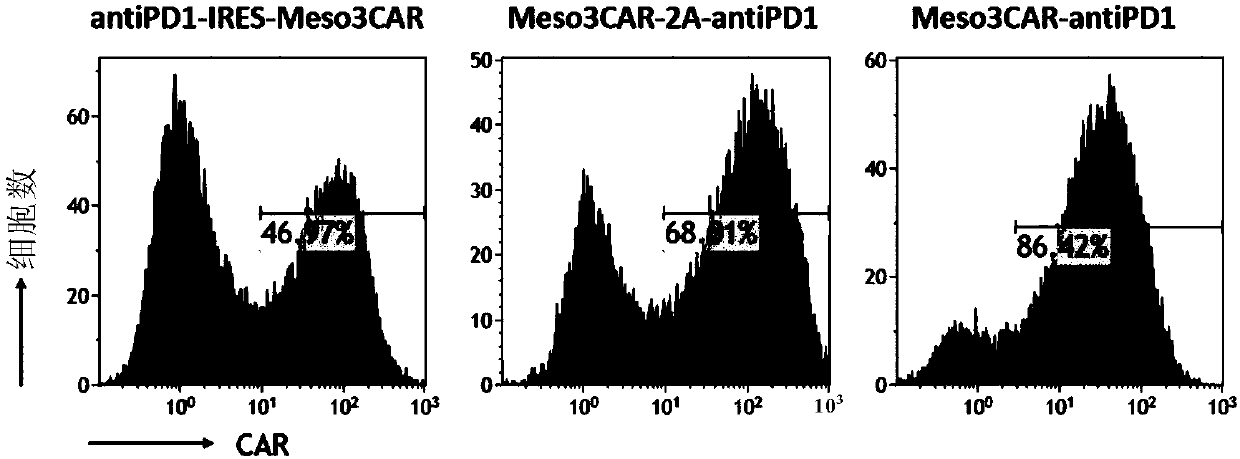
[0106] Example 3: Comparing the positive rate and antibody secretion of three kinds of mesothelin-targeted CAR T cells expressing PD1 antibody
[0107] 1. Positive rate of CAR T cells detected by flow cytometry
[0108] Collect the meso3CAR-antiPD1T cells, meso3CAR-2A-antiPD1T cells, and antiPD1-IRES-meso3CAR T cells obtained in the examples, and divide each into two parts, each with 1×10 6 For each cell, wash twice with normal saline, resuspend the cells in 100ul normal saline, add 1ug of mesothelin-biotin to one part, and not add to the other, and incubate at 4°C for 30 minutes. Wash twice with normal saline, resuspend the cells with 100ul normal saline again, add 1ul streptomycin-PE antibody, and incubate at 4°C for 30 minutes. Washed twice with normal saline, tested on the machine, and only added secondary antibody as a control, the results are as follows: Figure 2A .
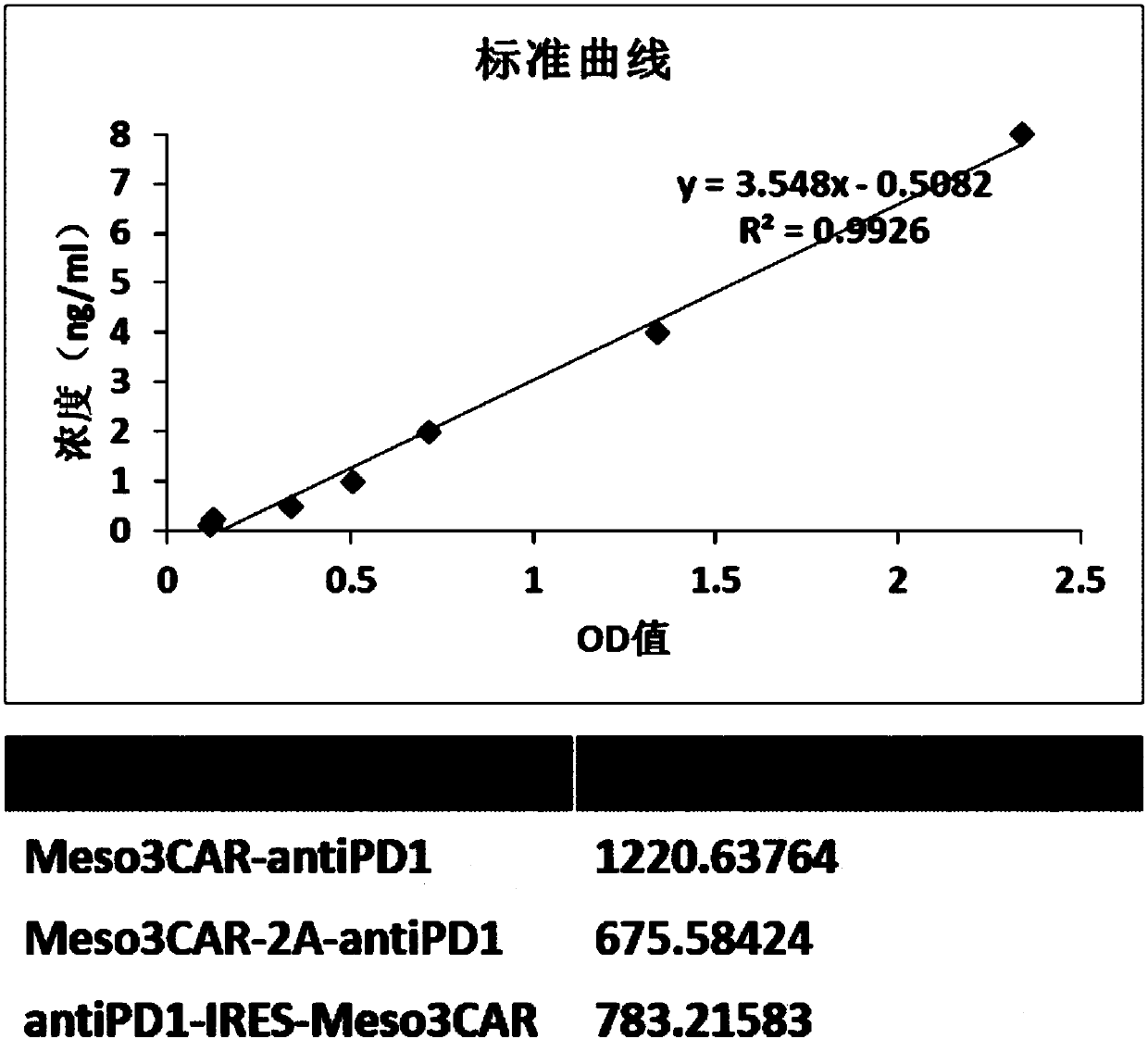
[0109] 2. ELISA detection of antiPD1 antibody expression levels of the three T cells
[0110] ① Dil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com