Application of photoacoustic probe in preparation of NTR photoacoustic detection reagent
A photoacoustic detection and probe technology, which is applied in measurement devices, material analysis through optical means, instruments, etc., can solve the problems of low resolution of fluorescent probes, unfavorable NTR accurate imaging analysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103] And the preparation method of the above photoacoustic probe of the present invention can be referred to as follows: NO 2 -R 1 -R 2 -Y 1 (i) with R 3 -Y 2 (ii) The target photoacoustic probe compound can be obtained by mixing reaction, the preparation method is relatively convenient, and the operation is relatively simple, which is suitable for large-scale expanded production;
[0104] Among them, in compound (i), R 1 C5~C30 arylene, heteroarylene, substituted arylene, or substituted heteroarylene; preferably, R 1 Arylene, heteroarylene, substituted arylene, or substituted heteroarylene of C5-C15; more preferably, R 1 C5-C15 heteroarylene or substituted heteroarylene, wherein the heteroatoms in the heteroarylene or substituted heteroarylene are one or more of nitrogen, oxygen, and sulfur, and each The number of heteroatoms contained in heteroaryl or substituted heteroarylene is one or more; for example, R 1 Can be, but not limited to: furan, thiophene, pyrrole, i...
Embodiment 1
[0139] (1) Preparation of MZ-BOC molecules:
[0140] Dissolve 2-nitroimidazole (0.5 g, 4.42 mmol) in 2 mL of DMF, and then add K 2 CO 3 (0.915g, 6.63mmol) and N-Boc-bromoethylamine (0.99g, 4.42mmol), reacted overnight under nitrogen protection;
[0141] Then, the solvent was removed by rotary evaporation, dried in vacuo, the obtained solid was dissolved in water, extracted with ethyl acetate, the organic phase was collected, the solvent was removed by rotary evaporation, and the crude product was recrystallized with ethyl acetate to obtain a dark yellow solid product MZ-BOC (0.10 g, 89%).
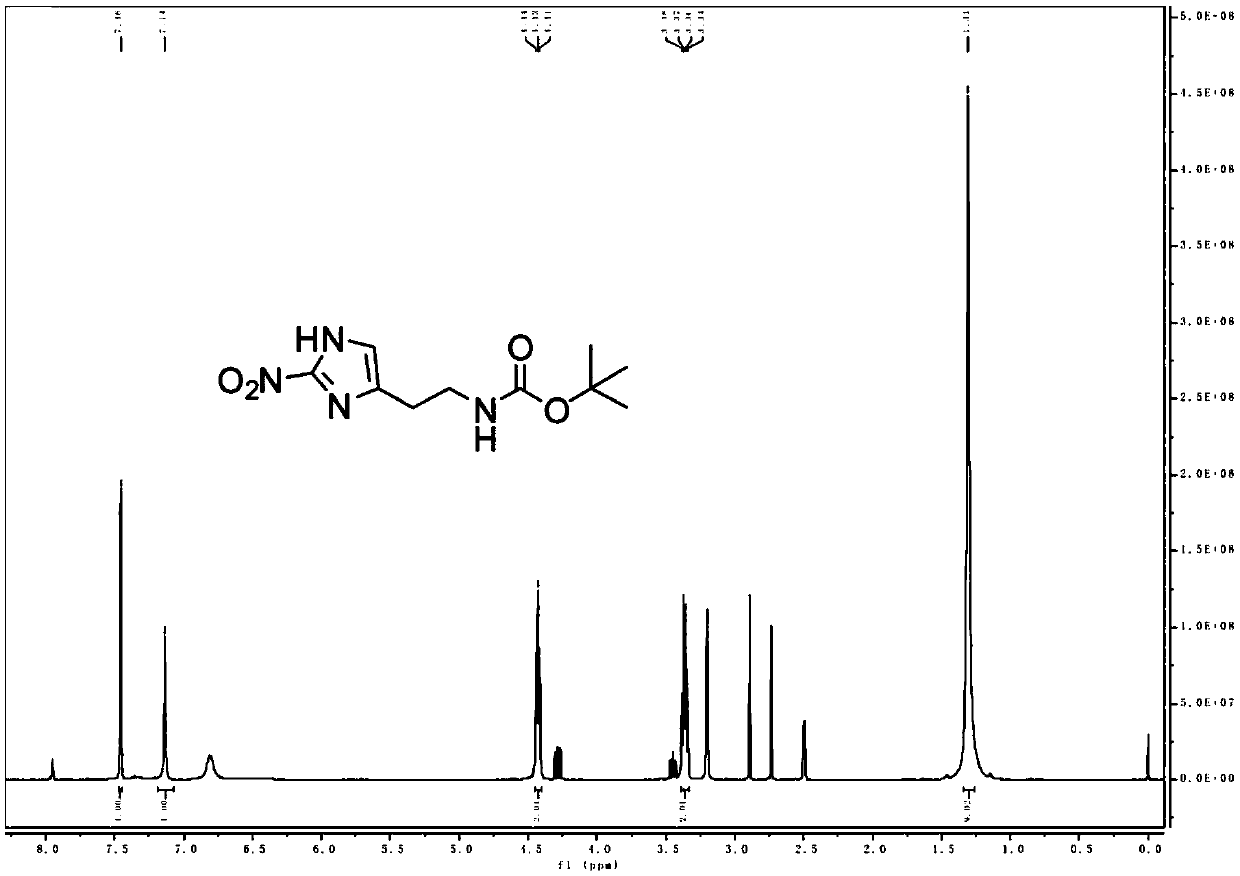
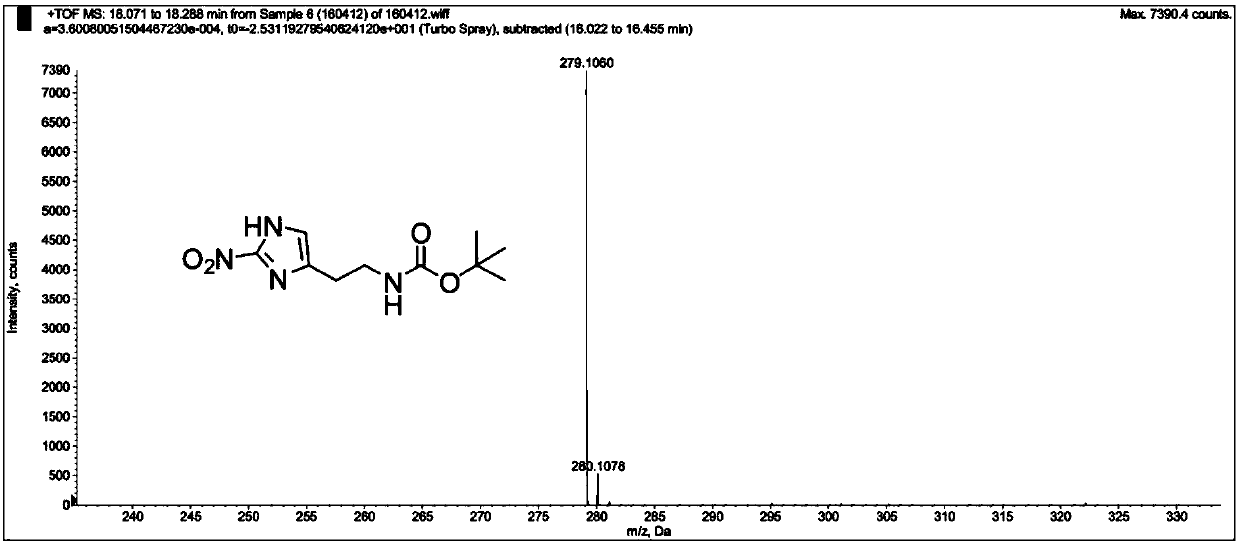
[0142] The product MZ-BOC NMR and mass spectrometry test patterns are respectively referred to figure 1 and figure 2 , the graph analysis is as follows:
[0143] MZ-BOC: 1 H NMR (400MHz, DMSO-d 6 )δ7.46(s, 1H), 7.14(s, 1H), 4.43(t, J=5.6Hz, 2H), 3.36(q, J=5.9Hz, 2H), 1.31(s, 9H);
[0144] HRMS (ESI + ): m / z calcd for C 10 h 16 N 4 o 4 :279.1064[M+Na +], found 279.1060.
[01...
Embodiment 2
[0159] (1) Preparation of MZ-BOC molecules:
[0160] 2-Nitroimidazole (1.0g, 8.84mmol) was dissolved in 4mL of DMF, and then K was added in batches under stirring 2 CO 3 (1.83g, 13.26mmol), and N-Boc-bromoethylamine (1.98g, 8.84mmol), reacted overnight under nitrogen protection.
[0161] The solvent was removed by rotary evaporation, and the obtained solid was dissolved in water, then extracted with ethyl acetate, the organic phase was collected, and the solvent was removed by rotary evaporation. The obtained crude product was recrystallized from ethyl acetate to obtain dark yellow solid product MZ-BOC (0.19g, 83%).
[0162] The product MZ-BOC NMR and mass spectrometry detection spectrum and analysis results are the same as those in Example 1.
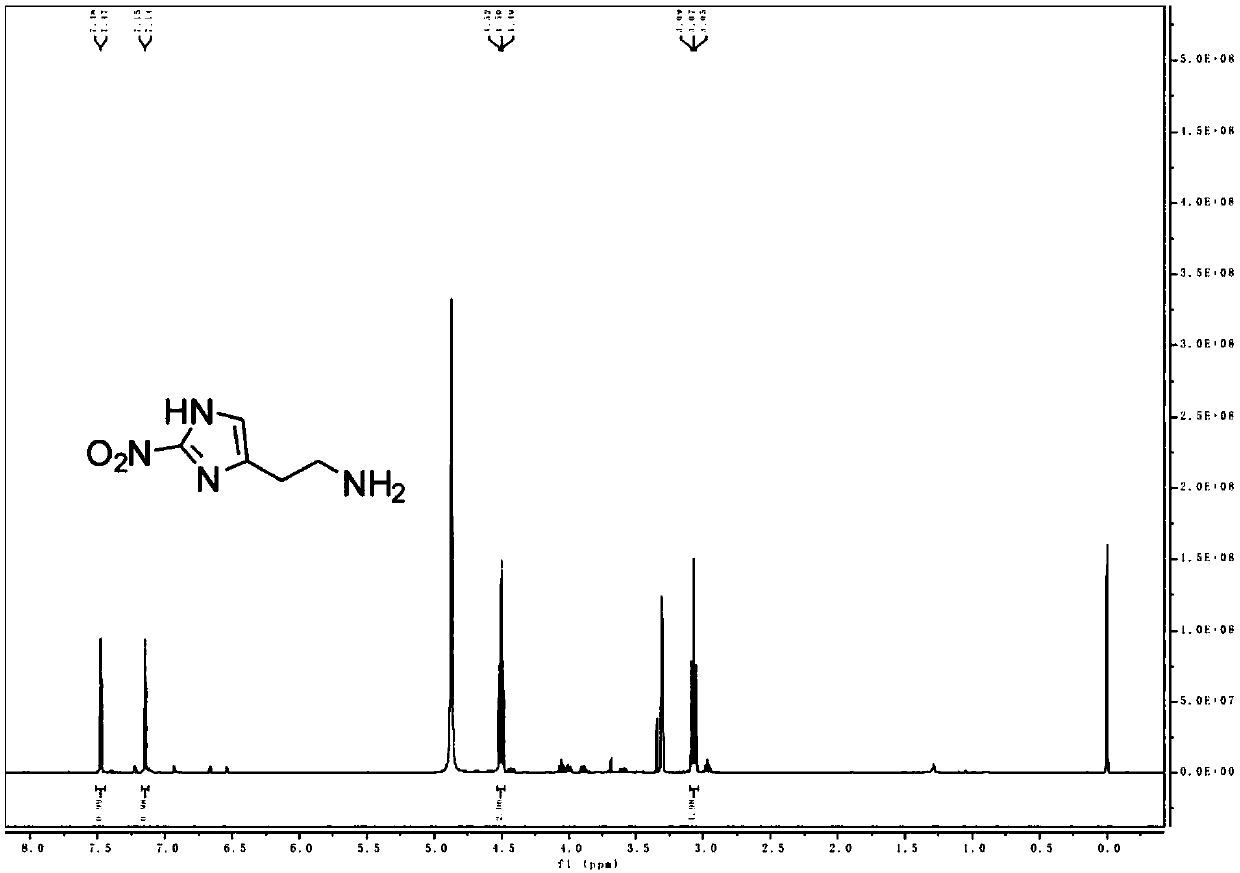
[0163] (2) Preparation of MZ molecules
[0164] Dissolve MZ-BOC (1.7g, 6.6mmol) in methanol (4mL), add 1.25M HCl in methanol (4mL) with stirring, and react at room temperature for 8h;
[0165] The solvent was removed by rotary eva...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com