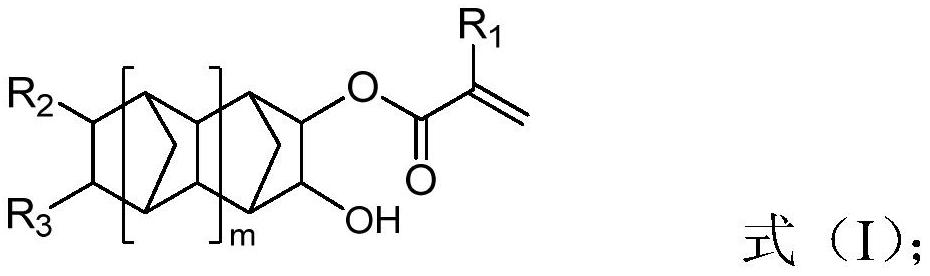
Acrylic ester compound for coating, preparation method, coating containing same and use
A technology of acrylates and compounds, which is applied in the preparation of organic compounds, carboxylate esters, chemical instruments and methods, etc., can solve the problems of difficult large-scale production, harsh synthesis conditions, and low yields, and achieve good phase Solubility, easy control of conditions, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] A kind of acrylate compound 3-hydroxybicyclo[2.2.1]heptyl-2-acrylate for coating Its preparation method comprises the following steps:
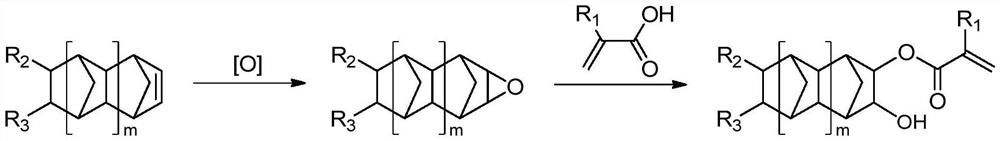
[0083] (1) Bicyclo[2.2.1]-2-heptene The epoxidation reaction:
[0084]Drop into 50.0g (0.532mol) bicyclo[2.2.1]-2-heptene, 11.7g sodium carbonate and 200g chloroform in a four-necked glass flask equipped with constant pressure dropping funnel, thermometer and mechanical stirring, Place in an ice-water mixing bath, start stirring, mix evenly and keep the system temperature at 5-15°C, control the pH value of the system at 3.5-5.5, and put 234.2g of 18.3% peracetic acid (0.564mol) into the constant pressure dropping funnel The solution is slowly added dropwise to the reaction system, keeping the reaction temperature not exceeding 25°C, and the reaction is continued for 2 hours after the dropwise addition is completed;
[0085] After the reaction, the mixed solution was left to stand for stratification, the lower organic layer solutio...
Embodiment 2
[0095] A combination of acrylate compounds for coatings, which is isomer 3-hydroxy-6-methylbicyclo[2.2.1]heptyl-2-acrylate and 3-hydroxy-5-methylbicyclo[2.2.1]heptyl-2-acrylate Its preparation method comprises the following steps:
[0096] (1) 5-Methylbicyclo[2.2.1]-2-heptene The epoxidation reaction:
[0097] Put 54.0g (0.500mol) 5-methylbicyclo[2.2.1]-2-heptene, 5.50g sodium carbonate and 270g dichloromethane into a four-necked glass flask equipped with a constant pressure dropping funnel, a thermometer and mechanical stirring Methane, put it in an ice-water mixed bath, start stirring, mix evenly and keep the system temperature at 5-15°C, control the pH value of the system at 3.5-5.5, and put 241.5g of 20.5% peroxygen into the constant pressure dropping funnel Propionic acid (0.550mol) solution was slowly added dropwise to the reaction system, keeping the reaction temperature not exceeding 25°C, and the reaction was continued for 3 hours after the dropwise addition was...
Embodiment 3
[0107] A kind of acrylate compound 3-hydroxy-5,6-dimethylbicyclo[2.2.1]heptyl-2-acrylate for coating The preparation method comprises the following steps:
[0108] (1) 5,6-Dimethylbicyclo[2.2.1]-2-heptene The epoxidation reaction:
[0109] 122g (1.0mol) 5,6-dimethylbicyclo[2.2.1]-2-heptene, 6.33g potassium carbonate and 122g Dichloromethane, put it in an ice-water mixing bath, start stirring, mix evenly and keep the system temperature at 5-15°C, control the pH value of the system at 3.5-5.5, and put 633.3g of 12.0% dichloromethane into the constant pressure dropping funnel Slowly add peracetic acid (1.0mol) solution into the reaction system dropwise, keep the reaction temperature not exceeding 25°C, and continue the reaction for 5h after the dropwise addition is completed;
[0110] After the reaction, the mixed solution was left to stand for stratification, the lower organic layer solution was removed, washed with water to pH 6.5-7.0, filtered, and the solvent was distill...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com