Porous carbon material based on porous organic polymer structure and preparation method and application thereof
A technology for porous carbon materials and polymers, applied in the field of porous carbon materials, can solve the problems of poor conductivity of porous organic polymers and limit practical applications, and achieve the effects of high specific capacitance, good application prospects, and excellent cycle stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 A porous carbon material C-LNU-16 based on a porous organic polymer structure
[0034] 1. Synthesis of Precursor LNU-16
[0035] Under nitrogen, 531 mg (0.827 mmoL) of 9,9-dioctylfluorene-2,7-diboronic acid pinacol ester was mixed with 303 mg (0.555 mmoL) of 2,4,6-tri-(4-bromo Phenyl)-[1,3,5]triazine was added into a round-bottomed flask containing 60mL of DMF solution, then frozen with liquid nitrogen, pumped with an oil pump and then thawed repeatedly for three times, and then 5mL of 2mol / L potassium carbonate aqueous solution and 40 mg tetrakis(triphenylphosphine)palladium were added to the reaction system. After repeating the above degassing process three times, the reaction system was heated to 130° C. and reacted for 2 days.
[0036] 2. Post-processing of precursor LNU-16
[0037] After the reaction was completed, the reactant was suction filtered to leave solid insoluble matter, which was washed with tetrahydrofuran, water and acetone solvents for m...
Embodiment 2
[0042] Example 2 A porous carbon material C-LNU-17 based on a porous organic polymer structure
[0043]In Example 1, 531mg (0.827mmoL) of 9,9-dioctylfluorene-2,7-diboronic acid pinacol ester was replaced by 371mg (0.831mmoL) of 9,9-dimethyl-2,7- For bis(borpinacol ester)fluorene, other steps were repeated in Example 1, and the obtained light green powder was the precursor porous organic polymer LNU-17 of the present invention.
[0044] The reaction equation of the precursor porous organic polymer LNU-17 is as follows:
[0045]
[0046] The sample obtained after carbonizing the precursor is the porous carbon material C-LNU-17 described in the present invention.
Embodiment 3
[0047] Example 3 A porous carbon material C-LNU-18 based on a porous organic polymer structure
[0048] In Example 1, 531mg (0.827mmoL) of 9,9-dioctylfluorene-2,7-diboronic acid pinacol ester was replaced with 346mg (0.555mmoL) of tris (4-boric acid pinesol ester phenyl)amine , other steps were repeated in Example 1, and the obtained bright green powder was the precursor porous organic polymer LNU-18 described in the present invention.
[0049] The reaction equation of the precursor porous organic polymer LNU-18 is as follows:
[0050]
[0051] The sample obtained after carbonizing the precursor is the porous carbon material C-LNU-18 described in the present invention.
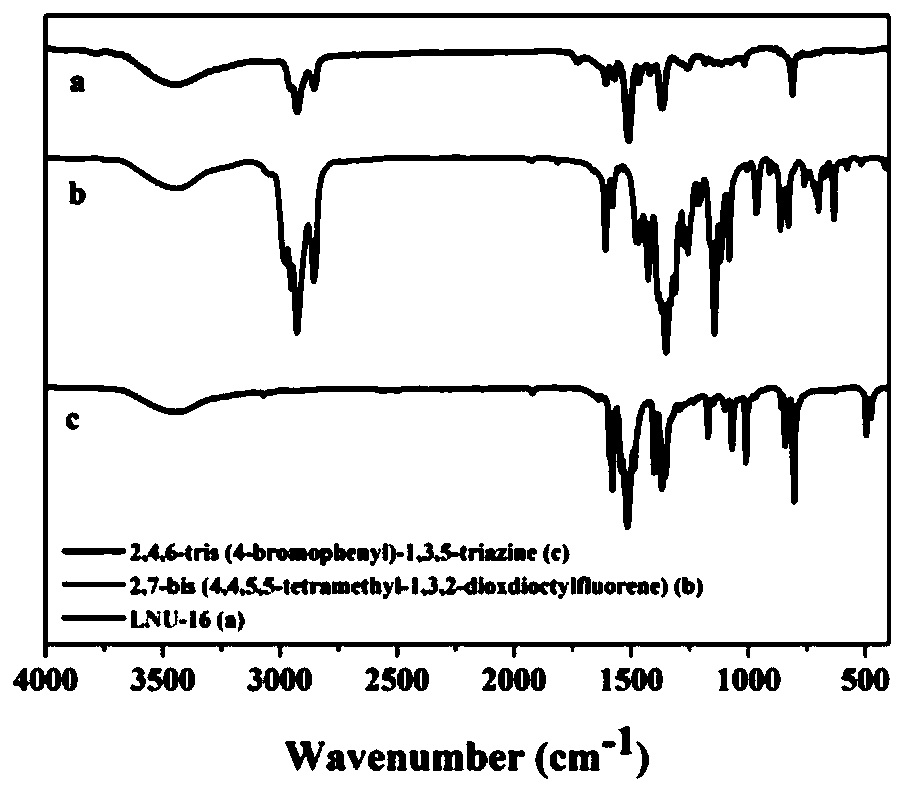
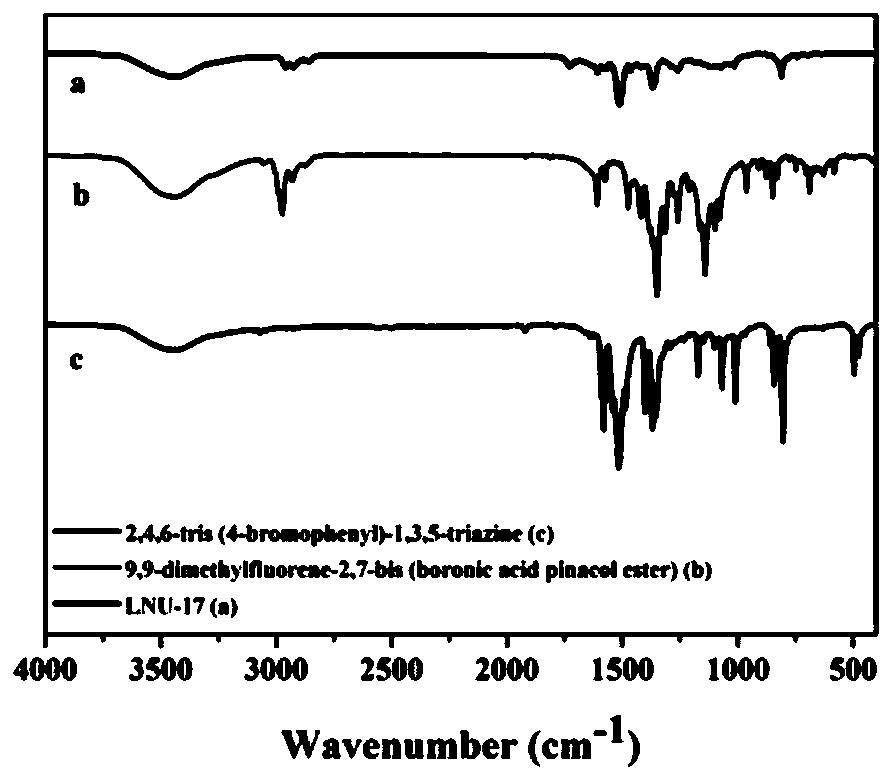
[0052] Such as Figure 1-1 to Figure 1-3 As shown, the precursor porous organic polymer LNU-16 (a), LNU-17 (b), LNU-18 (c) and the infrared spectrograms of the corresponding monomers prepared by the embodiment of the present invention 1-3, each The curve a in the figure is the infrared spectrum of the po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com