Synthesizing method of norfloxacin, ciprofloxacin and enrofloxacin
A technology of ciprofloxacin and norfloxacin, which is applied in the direction of organic chemistry, can solve the problems of low selectivity of the main reaction and high proportion of by-products substituted by six-position fluorine, and achieve low production cost, shortened reaction time, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
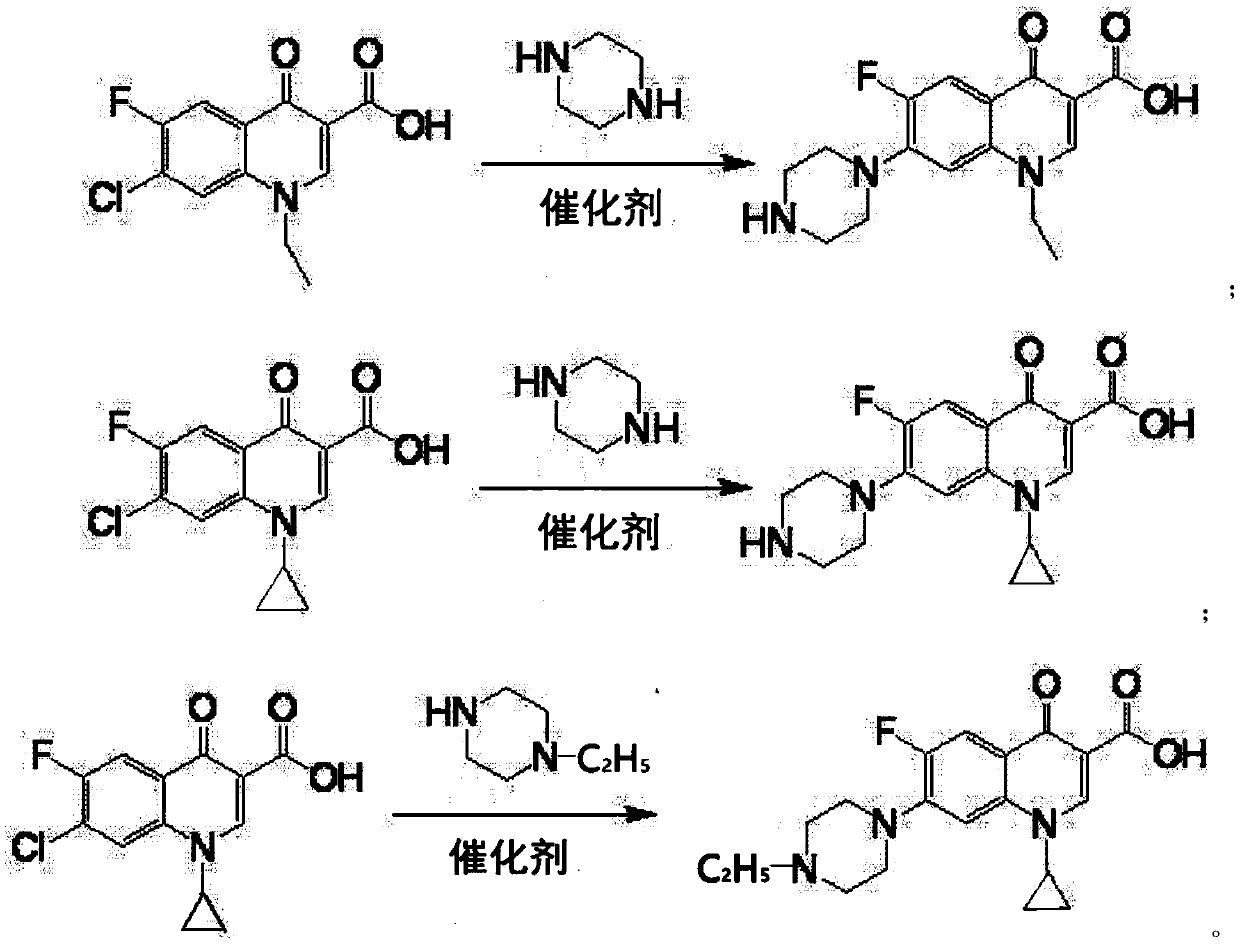
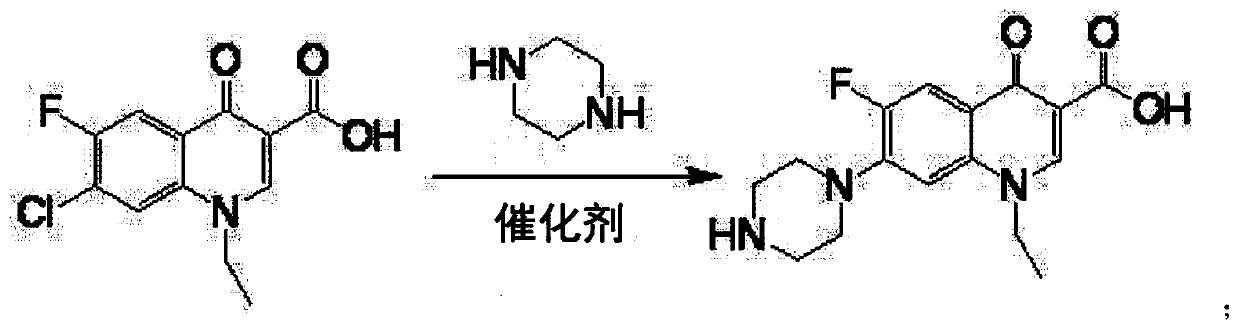
[0017] Put 100g of 1-ethyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid into a four-neck flask, piperazine 160g, water 20g, aluminum tribromide 2g, heat up to 40°C, keep warm for 9h, add 30g of sodium hydroxide, depressurize to dryness to recover piperazine, then add 400g of water to the residue to dissolve, add 0.2g of activated carbon, decolorize at 70°C for 30 minutes, filter, add the filtrate Adjust the pH to neutral with hydrochloric acid, cool to room temperature and filter to obtain the crude product of norfloxacin, then recrystallize with ethanol, filter and dry to obtain the finished product of norfloxacin 108g, the molar yield liquid phase monitoring purity 99.72%, 6 fluorine substitution impurity 0.16 %, molar yield 91.0%.
Embodiment 2
[0019] Put 100g of 1-ethyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid into a four-neck flask, piperazine 125g, water 30g, aluminum tribromide 1.5g, heat up to 65°C, keep warm for 5h, add 30g of sodium hydroxide, decompress to dryness to recover piperazine, then add 400g of water to the residue to dissolve, add 0.2g of activated carbon, decolorize at 70°C for 30 minutes, filter, and the filtrate Add hydrochloric acid to adjust the pH to neutral, cool to room temperature and filter to obtain the crude product of norfloxacin, then recrystallize with ethanol, filter and dry to obtain the finished product of norfloxacin 107g, the molar yield liquid phase monitoring purity 99.76%, 6 fluorine substitution impurities 0.13%, molar yield 90.3%.
Embodiment 3
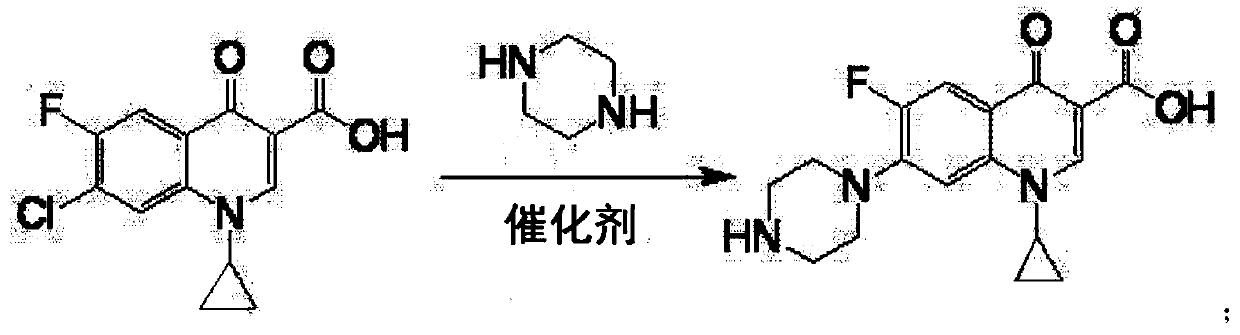
[0021] Put 100g of 1-ethyl-6fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, 150g of piperazine, 40g of water, and 3g of zinc bromide into a four-neck flask , heat up to 90°C, keep warm for 3 hours, add 30g of sodium hydroxide, decompress to dryness to recover piperazine, then add 400g of water to the residue to dissolve, add 0.2g of activated carbon, decolorize at 70°C for 30 minutes, filter, add hydrochloric acid to the filtrate Adjust the pH to neutral, cool to room temperature and filter to obtain the crude product of norfloxacin, then recrystallize with ethanol, filter and dry to obtain the finished product of norfloxacin 105g, the molar yield liquid phase monitoring purity 99.0%, 6 fluorine substitution impurity 0.5% , The molar yield is 87.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com