Phenyl dilactone compounds and uses in preparation of anti-complement drugs
A compound and dilactone technology, applied in the field of traditional Chinese medicine pharmacy, can solve problems such as excessive activation of the autoimmune system, and achieve the effect of good anti-complement activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
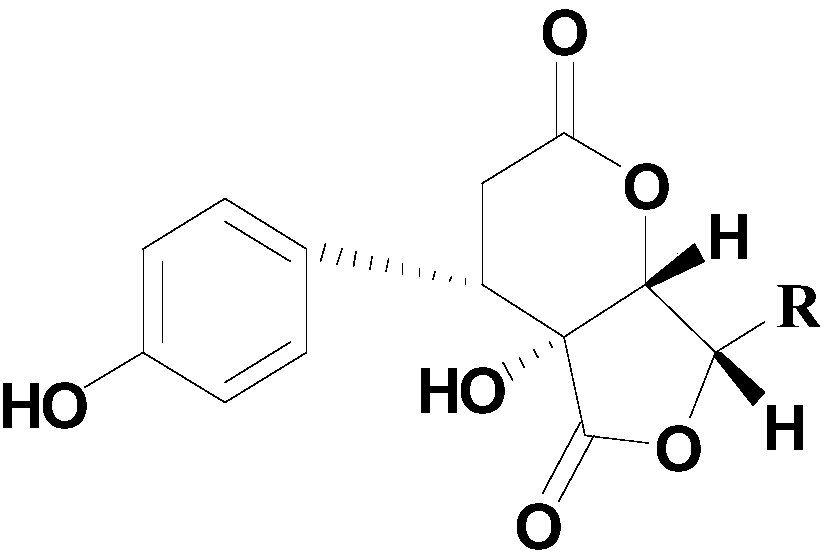
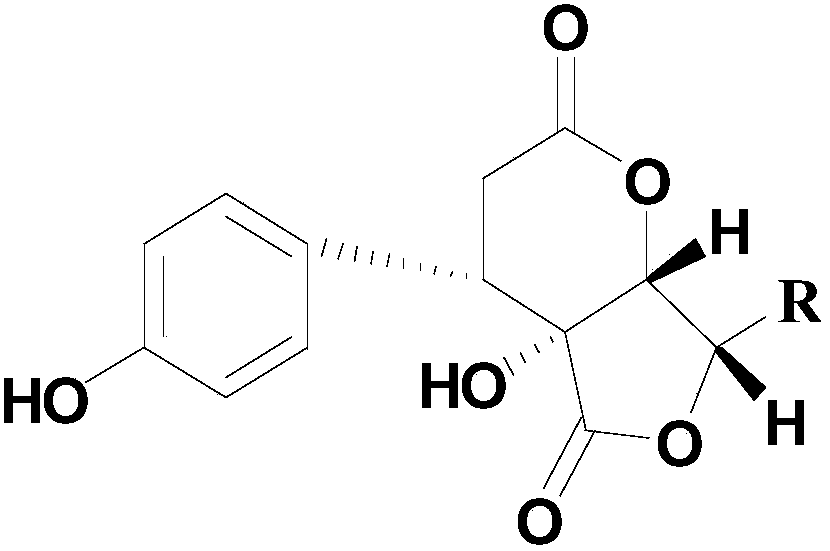
[0027] Example 1. Preparation of phenyl dilactone compounds
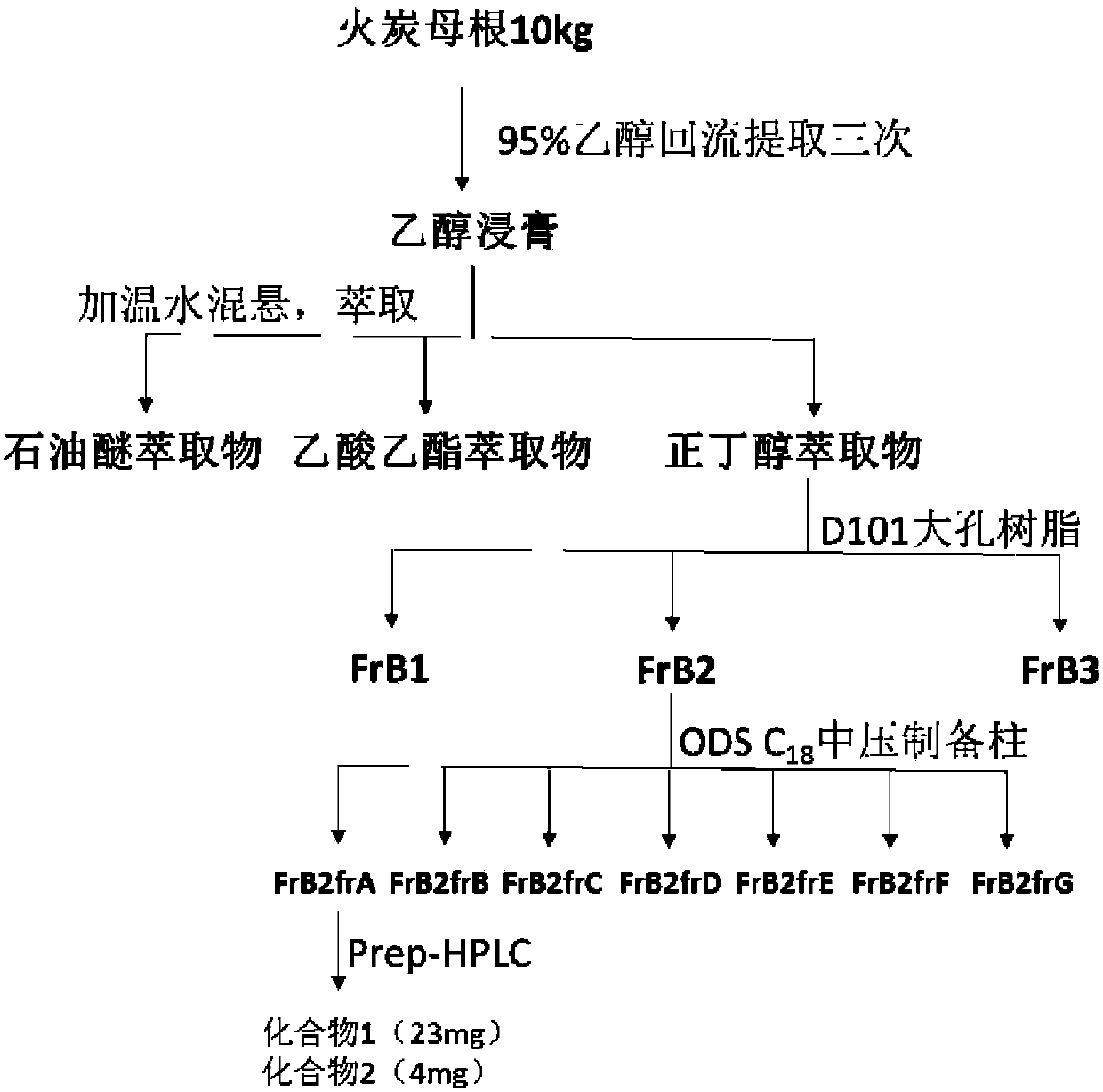
[0028] Take 10kg of Polygonum chinense Linn., crush it, and extract with 95% ethanol for 3 times. The extract is concentrated and suspended in water, and then extracted with petroleum ether, ethyl acetate and n-butanol to obtain the active part. 300g of butanol, take 250g of the extract and wet process for the macroporous resin D101 with H 2 O / EtOH (30, 60, 95%, v / v) obtained three fractions Fr.B1-Fr.B3. The active fraction passes through ODS C 18 Medium pressure rapid preparation column with CH 3 OH-H 2 O(25:75 to 100:0) eluted to obtain 7 Fr.B2fractions(AG). All fractions were purified by semi-preparative column, and compound maysedilactone C(1) and compound maysedilactone D(2) were obtained from Fr.B2fraction A. (CH 3 CN-H 2 O-CH 3 COOH 25:75:0.05);
[0029] After further identification, the NMR data of the obtained compounds 1 and 2 are shown in Table 1.
Embodiment 2
[0030] Example 2. In vitro anti-complement classical pathway experiment
[0031] Take 0.1 mL of complement (guinea pig serum), add barbital buffer (BBS) to make a 1:10 solution, and use BBS to double-dilute it to 1:20, 1:40, 1:80, 1:160, 1: 320, 1:640 and 1:1280 solutions. Dissolve 0.1 mL each of 1:1000 hemolysin, each concentration of complement and 2% sheep red blood cells (SRBC) in 0.3 mL BBS, mix them, and put them in a low-temperature high-speed centrifuge after a water bath at 37°C for 30 minutes at 5000 rpm and 4°C Centrifuge for 10 min. Take 0.2mL of supernatant from each tube to 96-well plate, and measure the absorbance at 405nm. The experiment also set up a complete hemolysis group (0.1mL 2% SRBC dissolved in 0.5mL tri-distilled water). The absorbance of the three-distilled water-soluble blood vessel was used as the standard for total hemolysis to calculate the hemolysis rate. Plot the complement dilution on the x-axis and the percentage of hemolysis on the y-axis. ...
Embodiment 3
[0032] Example 3. In vitro anti-complement alternative pathway experiment
[0033] Take 0.2mL of complement (human serum), add AP diluent (barbital buffer, pH 7.4, containing 5mM Mg 2+ , 8mMEGTA) after diluting it to 8 concentration gradients (1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256), add hemolysis In the reaction system, 0.15 mL of each concentration of complement and 0.2 mL of 0.5% RRBC were dissolved in 0.35 mL of AP diluent, mixed well, placed in a low-temperature high-speed centrifuge at 37°C for 30 minutes, and centrifuged at 5000 rpm, 4°C for 10 minutes. Take 0.2mL of supernatant from each tube to 96-well plate and measure the absorbance at 405nm. The experiment also set up a complete hemolysis group (0.5% RRBC 0.2mL dissolved in 0.5mL tri-distilled water). The absorbance of the three-distilled water-soluble blood vessel was used as the standard for total hemolysis to calculate the hemolysis rate. Take the complement dilution factor as the x-axis, and plot the perc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com