Chimeric protein, immune effector cell expressing chimeric protein and application thereof
A technology of immune effector cells and chimeric proteins, applied in the field of chimeric proteins and genetically engineered immune effector cells, can solve the problems of lack of immune cell proliferation and surviving immune effector cells, and insignificant efficacy enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1. Construction of vectors expressing chimeric proteins
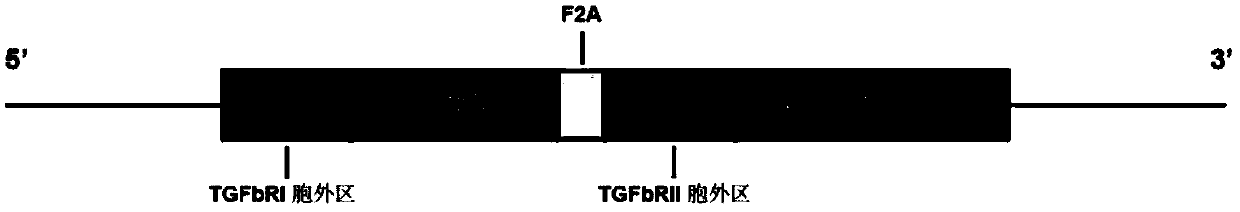
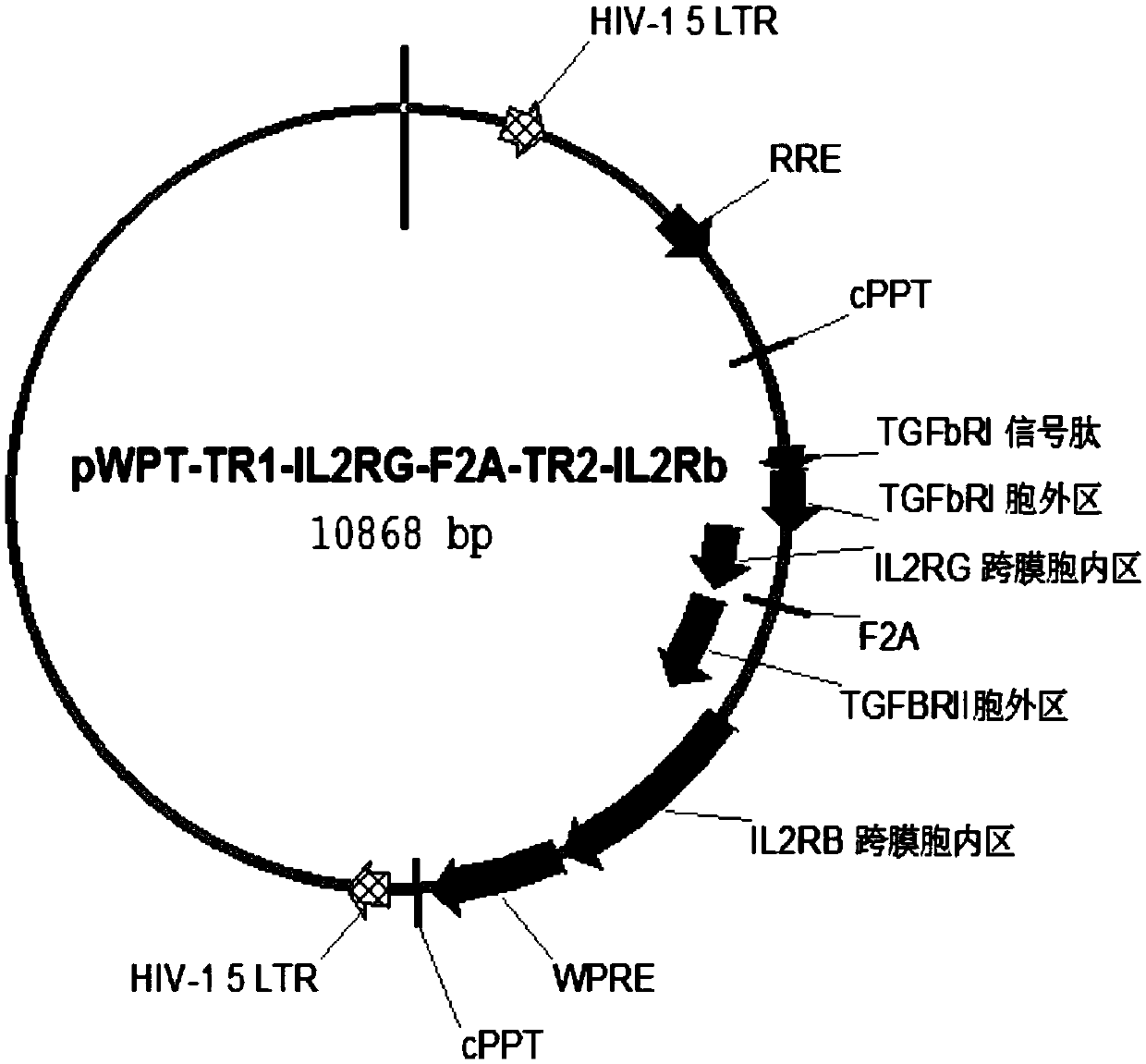
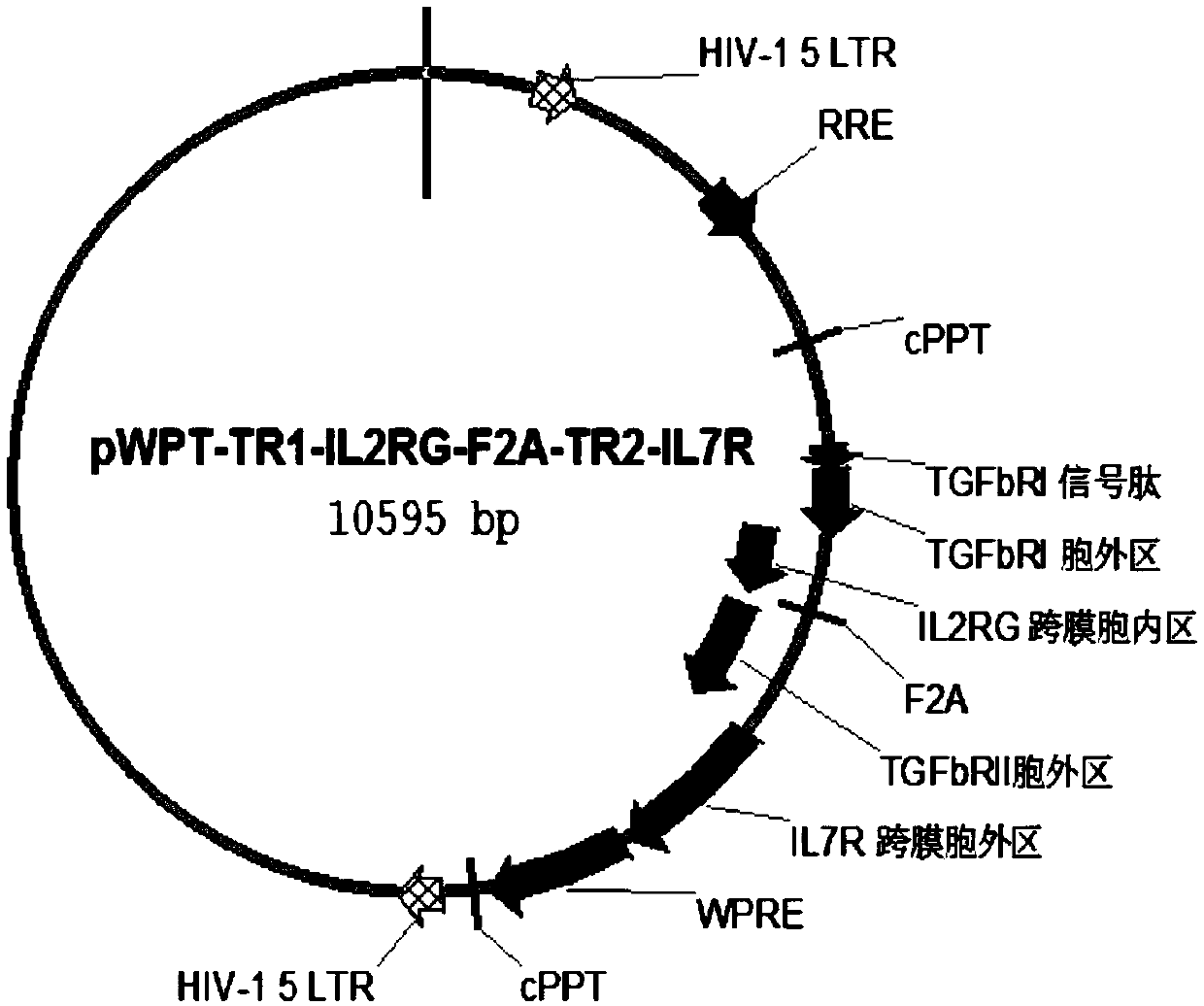
[0133] Follow the steps below to build a build that includes figure 1 Plasmids expressing chimeric proteins of the indicated constructs.
[0134] (1) Human TGF-β receptor I signal peptide (SEQ ID NO: 2), extracellular domain of TGF-β receptor I (SEQ ID NO: 4) and human IL-2RG transmembrane region (SEQ ID NO: 6), the coding nucleotide sequence of the IL-2RG intracellular domain (SEQ ID NO:8) is connected, and the nucleotide sequence (SEQ ID NO:37) of the first chimeric protein chIL2RG is constructed, and its coded amino acid sequence is as shown in SEQ IDNO:27).
[0135] (2) Human TGF-β receptor II signal peptide (SEQ ID NO:10), TGF-β receptor II extracellular domain (SEQ ID NO:12) and human IL-2Rβ transmembrane region (SEQ ID NO:14 ), the coding nucleotide sequence of IL-2Rβ intracellular region (SEQ ID NO: 16) was connected to construct the nucleotide sequence (SEQ ID NO: 38) of the second chimeric pr...
Embodiment 2
[0139] Example 2. Determination of T cell phosphorylation level
[0140] The constructed lentivirus plasmid 1, lentivirus plasmid 2, and lentivirus plasmid 3 were respectively co-transfected with lentivirus packaging plasmids into HEK-293T cells to prepare corresponding lentiviruses, which are respectively denoted as lentivirus 1, lentivirus 2, lentivirus 2, Lentivirus3.
[0141] Human PBMC were cultured in AIM-V medium, added with 2% human AB serum, 500 U / mL recombinant human IL-2, and activated with CD3 / CD28 antibody combined with magnetic beads for 48 hours to obtain activated T cells. After T cells were activated, they were infected with lentivirus 1, lentivirus 2, and lentivirus 3, respectively, to obtain T cells chTR2, chTR7, and chTR21 expressing chimeric proteins.
[0142] Remove serum and rest for 24 hours, take blank T cell UTD (untransfected T cell, Untransfected) as blank control, use UTD, chTR2, chTR7, chTR21 cells, divide into TGF-β1 positive group (+) and negat...
Embodiment 3
[0144] Example 3. Effects of T cells expressing chimeric proteins on the differentiation level of Treg under the stimulation of recombinant human TGF-β1
[0145] Human PBMC were cultured in AIM-V medium, and 2% human AB serum and 500 U / mL recombinant human IL-2 were added to obtain the corresponding cell culture medium. The T cells chTR7 and chTR21 obtained in Example 2 and the empty control T cell UTD were cultured in the above cell culture medium, treated with recombinant human TGF-β1 (5 ng / mL) and cultured for 4 days, and the cells were collected to label Treg markers with antibodies CD4, CD25, and Foxp3 were detected by flow cytometry, and the test group not treated with recombinant human TGF-β1 was used as a control (CTRL). The result is as Figure 4A and 4B As shown, UTD cells stimulated by TGF-β1 Foxp3 + CD4 + CD25 + The proportion of the population increased, indicating that TGF-β1 induced Treg differentiation, while the Foxp3 of the cells in the chimeric receptor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com