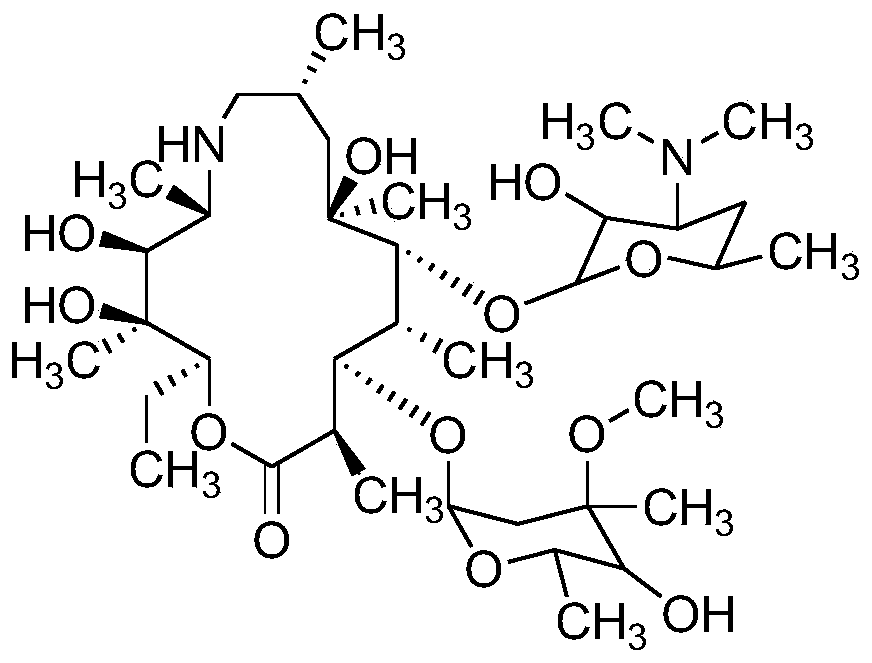
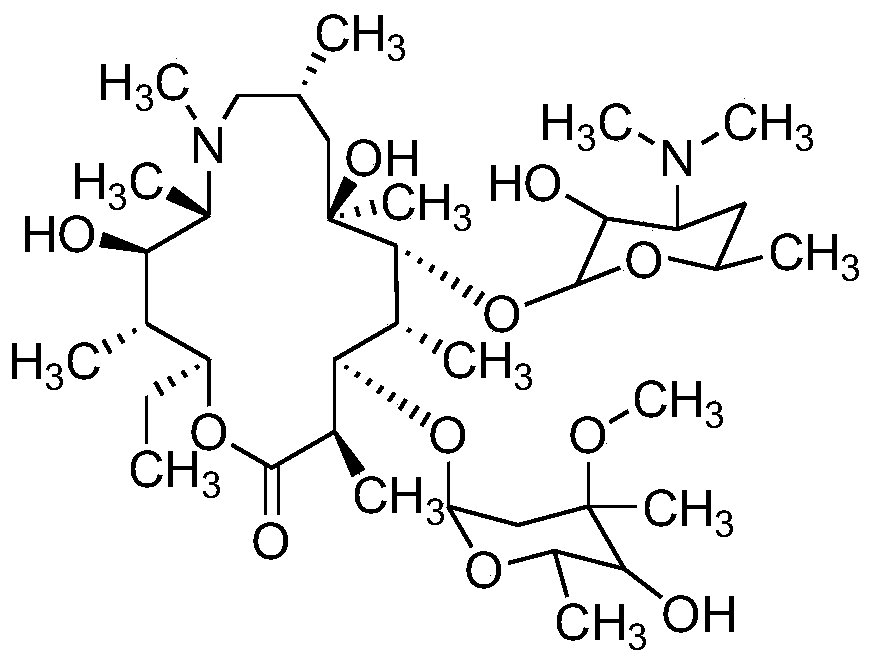
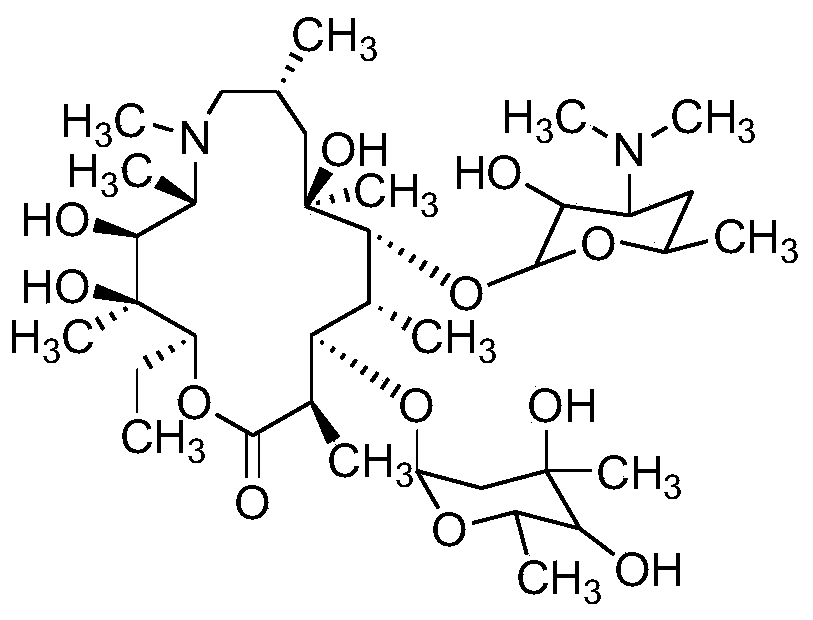
Method for measuring related substances of azithromycin capsule by high performance liquid chromatography
A technology of high-performance liquid chromatography and azithromycin gel, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as the inability to realize the determination of azithromycin capsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0041] Experimental Example 1 System suitability test
[0042] Preparation of mixed control solution: take appropriate amount of impurities A, B, C, E, F, G, H, I, J, L, M, N, Q, R, S and azithromycin, add diluent (diluent is the volume ratio) It is a 7:7:6 ammonium dihydrogen phosphate solution (weigh 1.73 g of ammonium dihydrogen phosphate, add water to dissolve and dilute to 1000 mL, adjust the pH value to 10.0±0.1 with ammonia test solution, a mixture of methanol and acetonitrile,) dissolve and dissolve Dilute to prepare a solution containing 10 mg of azithromycin per 1 mL and 0.05 mg of each impurity as a mixed control solution.
[0043] The preparation of each impurity positioning solution: take the appropriate amount of impurities A, B, C, E, F, G, H, I, J, L, M, N, Q, R, S, respectively add the diluent to dissolve and dilute to make Each 1 mL solution containing 0.05 mg of the above impurities was used as the impurity positioning solution.
[0044] Preparation of the...
experiment example 2
[0054] Experimental example 2 Linearity and range test
[0055] Stock solution of each impurity solution: Precisely weigh 10 mg each of impurities A, B, C, E, F, G, H, I, J, L, M, N, Q, R, and S, and place them in different 5mL volumetric flasks, respectively. Add an appropriate amount of diluent, ultrasonically dissolve it, add diluent to dilute to the mark, shake well, and use as impurities A, B, C, E, F, G, H, I, J, L, M, N, Q, R, S solution stock solution.
[0056] Azithromycin reference substance stock solution: Accurately weigh 10 mg of azithromycin reference substance, put it in a 5mL volumetric flask, add an appropriate amount of diluent, dissolve by ultrasonic, add diluent to dilute to the mark, and shake well.
[0057] Linear solution: Precisely measure 0.5 mL of azithromycin reference stock solution, 0.5 mL of impurity B solution stock solution, 0.1 mL of impurity G solution stock solution, and impurities A, C, E, F, H, I, J, L, M, N 0.25mL of each of the stock so...
experiment example 3
[0077] Experimental example 3 recovery rate test
[0078] Test solution: Accurately weigh an appropriate amount of azithromycin capsule content (equivalent to a prescription ratio of 250 mg of azithromycin), put it in a 25mL volumetric flask, add an appropriate amount of diluent, ultrasonicate for 10 minutes to dissolve azithromycin, let it cool, and add diluent to dilute to the mark , Shake well, filter, take the filtrate, that is. (New system for temporary use)
[0079] Stock solution of each impurity solution: Precisely weigh 10 mg each of impurities A, B, C, E, F, G, H, I, J, L, M, N, Q, R, and S into different 5mL volumetric flasks, respectively. Add an appropriate amount of diluent, ultrasonically dissolve it, add diluent to dilute to the mark, shake well, and use as impurities A, B, C, E, F, G, H, I, J, L, M, N, Q, R, S solution stock solution.
[0080] Recovery rate reference solution stock solution: Precisely measure the above impurity B solution stock solution 0.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com