Method for separating oseltamivir phosphate and oseltamivir phosphate SSR-isomers through normal-phase chromatography method
A technology of oseltamivir phosphate and enantiomers, which is applied in the field of analytical chemistry, can solve the problems of no oseltamivir phosphate and the inability to separate oseltamivir phosphate well, and the method is simple and convenient , high sensitivity and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
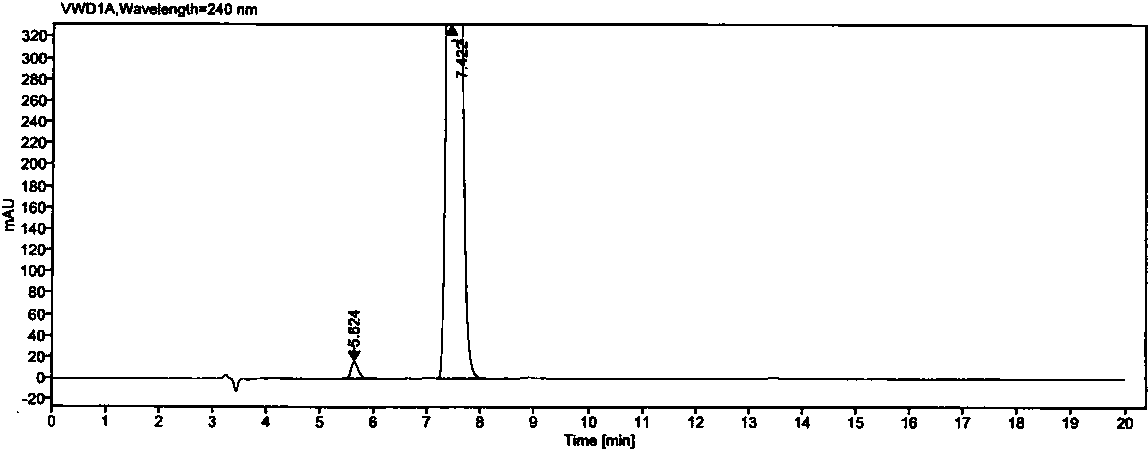
[0029] The separation and determination of oseltamivir phosphate and its enantiomers in the test sample of example 1
[0030] 1. Instruments and reagents:
[0031] Agilent 1260 liquid chromatograph equipped with G1314F ultraviolet detector and HPLC chromatographic workstation equipped with analytical instruments.
[0032] n-Hexane (HPLC), absolute ethanol (HPLC), methanol (HPLC), trifluoroacetic acid (TFA) (HPLC), diethylamine (AR)
[0033] 2. Chromatographic conditions:
[0034] Chromatographic column: the surface of silica gel is coated with cellulose-tris(3,5-dichlorophenylcarbamate) column (4.6mm×250mm, 5μm);
[0035] Detection wavelength: 240nm;
[0036] Flow rate: 0.9 ~1.1ml / min;
[0037] Column temperature: 33~37℃;
[0038] Injection volume: 25μl
[0039] Mobile phase: n-hexane-absolute ethanol-methanol-trifluoroacetic acid-diethylamine volume ratio is (90:8:2:0.4:0.2)
[0040] Diluent: n-hexane-absolute ethanol-methanol volume ratio 1:1:3
[0041] 3. Preparatio...
Embodiment 2
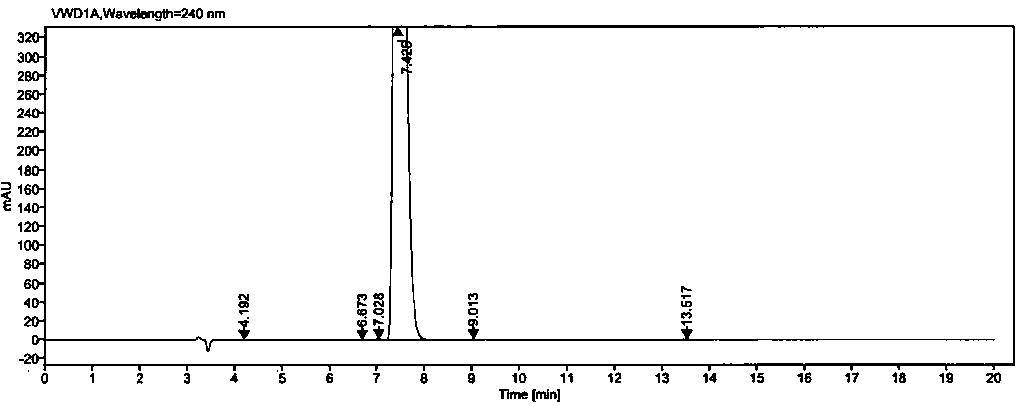
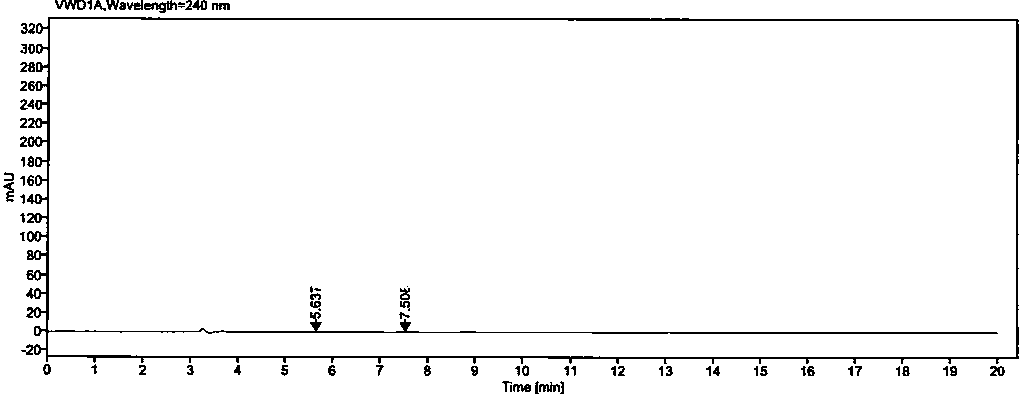
[0050] Example 2: Methodological Validation
[0051] 1. Quantitation limit and detection limit
[0052] Precisely measure 1ml of each of the solutions (1)~(2) under the preparation of the above-mentioned related solutions, put them in the same 100ml measuring bottle, dilute to the mark with diluent, shake well, and use it as the stock solution (A).
[0053] Detection limit solution: Accurately measure 1ml of the above stock solution (A), put it in a 50ml measuring bottle, dilute to the mark with a diluent, and shake well (equivalent to 0.02% of the concentration of the test solution).
[0054] Quantitative limit solution: Accurately measure 1ml of the above stock solution (A), put it in a 20ml measuring bottle, dilute to the mark with diluent, and shake well (equivalent to 0.05% of the concentration of the test solution).
[0055] Precisely measure 25 μl of each of the above detection limit and quantification limit solutions, inject them into the liquid chromatograph respecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com