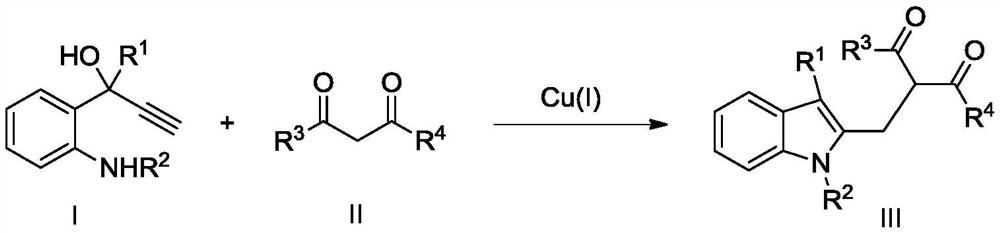
A kind of preparation method of 2,3-disubstituted indole compounds
An aniline compound and compound technology, applied in 2 fields, can solve problems such as by-products, and achieve the effects of mild reaction conditions, potential physiological activity, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
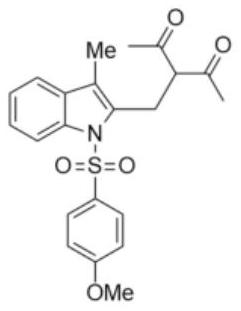
[0021] Preparation of 3-(2-(3-methyl-1-p-toluenesulfonyl-indole)-methyl)-2,4-pentanedione
[0022] 2-(3-Hydroxy-3-methyl-1-butyne)-N-p-toluenesulfonylanilide (0.1 mmol, 31.5 mg) was dissolved in 1,2-dichloroethane (2 mL) under air In, add acetylacetone (0.5mmol, 50mg) and Cu(CH 3 EN) 4 PF 6 (0.01mmol, 3.7mg), the reaction mixture was stirred at 60°C, and reacted for 4h. After the reaction, 30mg of white solid was obtained by column chromatography, with a yield of 76%.
[0023]
[0024] M.p.=103-105℃. 1 H NMR (400MHz, CDCl 3 ,TMS): δ8.20(d,J=8.0Hz,1H),7.51(d,J=8.0Hz,2H),7.37-7.26(m,3H),7.12(d,J=8.0Hz,2H) ,4.64(t,J=8.0Hz,1H),3.44(d,J=8.0Hz,2H),2.31(s,3H),2.19(s,6H),2.12(s,3H). 13 C NMR (100MHz, CDCl 3 ):δ203.4,144.9,136.9,135.1,132.7,131.5,129.8,126.2,124.9,123.9,120.1,119.0,115.4,66.5,30.8,25.6,21.5,9.2.HRMS(ESI-TOF)m / z calcd for C 22 h 23 NO 4 S(M+Na) + 420.1240,found 420.1239.
Embodiment 2
[0026] Preparation of 3-(2-(3-methyl-1-p-methoxybenzenesulfonyl-indole)-methyl)-2,4-pentanedione
[0027] 2-(3-Hydroxy-3-methyl-1-butyne)-N-p-methoxybenzenesulfonylaniline (0.1 mmol, 33.1 mg) was dissolved in 1,2-dichloroethane under air (2mL), then add acetylacetone (0.5mmol, 50mg) and Cu(CH 3 EN) 4 PF 6 (0.01mmol, 3.7mg), the reaction mixture was stirred at 60°C for 4h, and after the reaction, 32mg of a yellow solid was obtained by column chromatography, with a yield of 78%.
[0028]
[0029] Mp=135-137°C. 1 H NMR (400MHz, CDCl3, TMS): δ8.18 (d, J = 8.0Hz, 1H), 7.55 (d, J = 8.0Hz, 2H), 7.36-7.23 (m, 3H), 6.78 (d, J =8.0Hz, 2H), 4.63(t, J=8.0Hz, 1H), 3.75(s, 3H), 3.42(d, J=8.0Hz, 2H), 2.17(s, 6H), 2.10(s, 3H ). 13 C NMR (100MHz, CDCl 3 ): δ203.4, 163.7, 137.0, 132.7, 131.5, 129.5, 128.4, 124.9, 123.9, 120.1, 119.0, 115.4, 114.3, 66.5, 56.6, 30.8, 25.6, 9.2. HRMS (ESI-TOF) m / z calcd for C 22 h 23 NO 5 S(M+Na) + 436.1189,found 436.1191.
Embodiment 3
[0031] Preparation of 3-(2-(3-methyl-1-(2,4,6-trimethylbenzenesulfonyl-indole)-methyl)-2,4-pentanedione
[0032] Under air, 2-(3-hydroxy-3-methyl-1-butyne)-N-(2,4,6-trimethylbenzenesulfonyl)aniline (0.1 mmol, 34.3 mg) was dissolved in 1 , in 2-dichloroethane (2mL), then add acetylacetone (0.5mmol, 50mg) and Cu(CH 3 EN) 4 PF 6 (0.01mmol, 3.7mg), the reaction mixture was stirred at 60°C, reacted for 4h, and separated by column chromatography after the reaction to obtain 32mg of a yellow liquid with a yield of 75%.
[0033]
[0034] 1 H NMR (400MHz, CDCl 3 ,TMS):δ7.48(d,J=8.0Hz,1H),7.42(d,J=8.0Hz,1H),7.19-7.13(m,2H),6.90(s,2H),4.45(t, J=8.0Hz, 1H), 3.37(d, J=8.0Hz, 2H), 2.32(s, 6H), 2.26(s, 3H), 2.14(s, 3H), 2.11(s, 6H). 13 C NMR (100MHz, CDCl3): δ203.5, 143.8, 139.6, 136.2, 134.8, 133.5, 132.3, 129.7, 124.3, 122.7, 119.1, 116.5, 113.5, 65.8, 30.8, 25.2, 22.1, 21.0, 8.9-HRMS (ESI TOF)m / z calcd for C24H27NO4S(M+Na) + 448.1553,found448.1554.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com