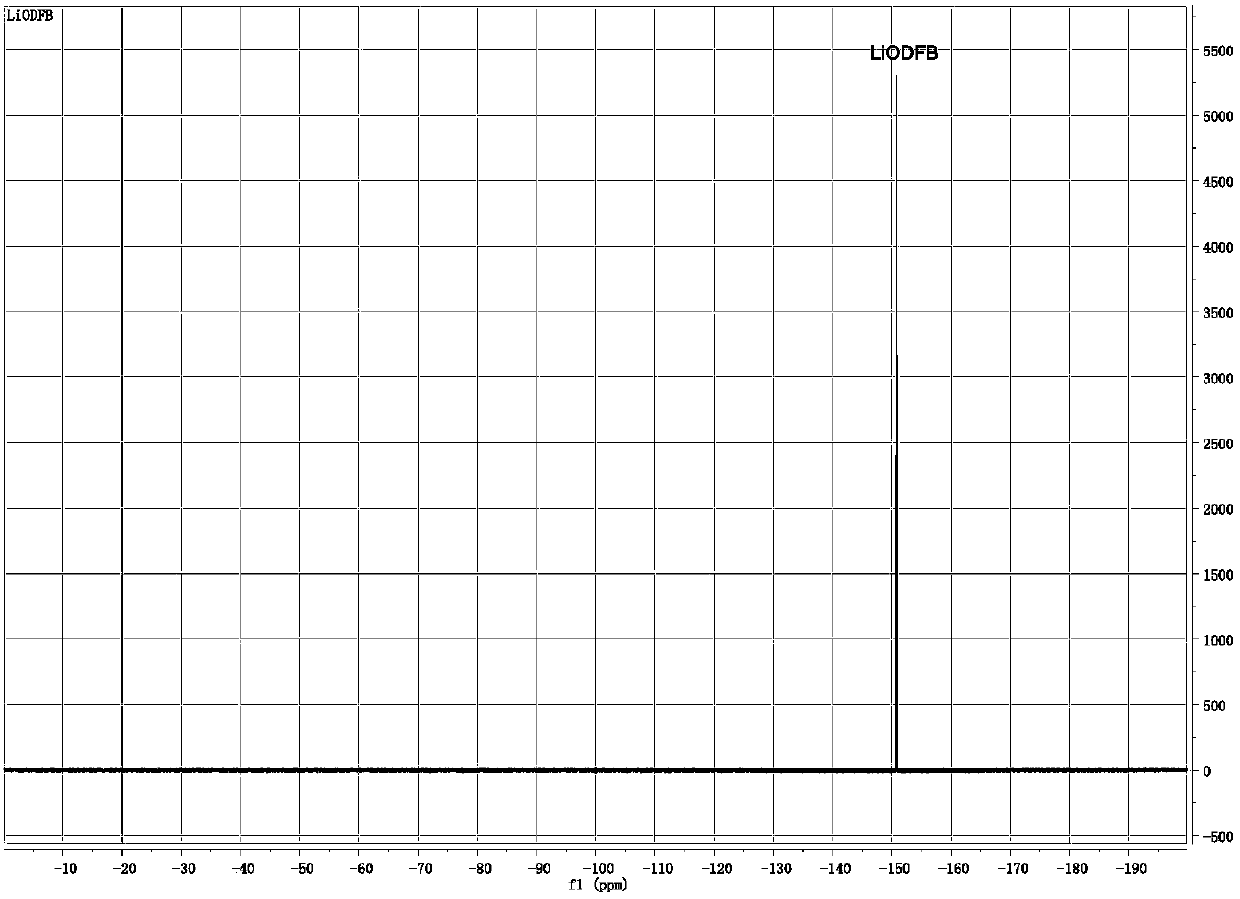
Preparation method of lithium difluoro(oxalato)borate
A technology of lithium difluorooxalate borate and lithium oxalate, applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problem of low purity of LiDFOB, unfavorable industrial production, and loss of reaction raw materials to avoid product loss, cheap materials, and easy access to materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Step 1: Under the protection of nitrogen, add 40g of lithium oxalate and 150mL of acetonitrile into a 500mL three-necked flask, and then dropwise add 120g of boron trifluoride in ether solution. Clear reddish-brown solutions of lithium difluorooxalate borate and lithium tetrafluoroborate;
[0047] Step 2: Under the protection of nitrogen, add 66g of potassium oxalate and 300mL of acetonitrile to a 1000mL three-necked flask, then dropwise add 120g of boron trifluoride in ether solution. After the addition, the temperature is raised to 85°C for reaction, and heated to reflux for 10 hours to obtain White suspension of potassium difluorooxalate borate and potassium tetrafluoroborate;
[0048] Step 3: Under the protection of nitrogen, slowly add the transparent reddish-brown solution obtained in step 1 to the white suspension obtained in step 2, and heat to reflux at 80°C for 18 hours;
[0049] Step 4: After the reaction is completed, filter, concentrate the filtrate to 200...
Embodiment 2
[0051] Step 1: Under the protection of nitrogen, add 40g of lithium oxalate and 150mL of dimethyl carbonate to a 500mL three-necked flask, then dropwise add 120g of boron trifluoride in ether solution, after the dropwise addition, raise the temperature to 80°C for reaction, and heat to reflux for 8 hours , to obtain a transparent reddish-brown solution containing lithium difluorooxalate borate and lithium tetrafluoroborate;
[0052] Step 2: Under the protection of nitrogen, add 99g of potassium oxalate and 500mL of dimethyl carbonate to a 1000mL three-necked flask, then dropwise add 180g of boron trifluoride in ether solution, after the dropwise addition, raise the temperature to 80°C for reaction, and heat to reflux for 12 hours , to obtain a white suspension containing potassium difluorooxalate borate and potassium tetrafluoroborate;
[0053] Step 3: Under the protection of nitrogen, slowly drop the transparent red-brown solution obtained in step 1 into the white suspension ...
Embodiment 3
[0056] Step 1: Under the protection of nitrogen, add 40g of lithium oxalate and 150mL of diethyl carbonate to a 500mL three-necked flask, then dropwise add 120g of boron trifluoride in ether solution, after the dropwise addition, raise the temperature to 70°C for reaction, and heat to reflux for 9 hours , to obtain a transparent reddish-brown solution containing lithium difluorooxalate borate and lithium tetrafluoroborate;
[0057] Step 2: Under the protection of nitrogen, add 82.5g of potassium oxalate and 400mL of diethyl carbonate to a 1000mL three-necked flask, then add 150g of boron trifluoride in ether solution dropwise, after the addition, heat up to 80°C for reaction, and heat to reflux for 11 hours, a white suspension containing potassium difluorooxalate borate and potassium tetrafluoroborate was obtained;
[0058] Step 3: Under the protection of nitrogen, slowly add the transparent reddish-brown solution obtained in step 1 to the white suspension obtained in step 2, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com