New method for xylanase secretory expression in escherichia coli
A technology of xylanase and Escherichia coli, which is applied in the field of genetic engineering to achieve the effect of maintaining high activity, less miscellaneous protein content, and being conducive to large-scale industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
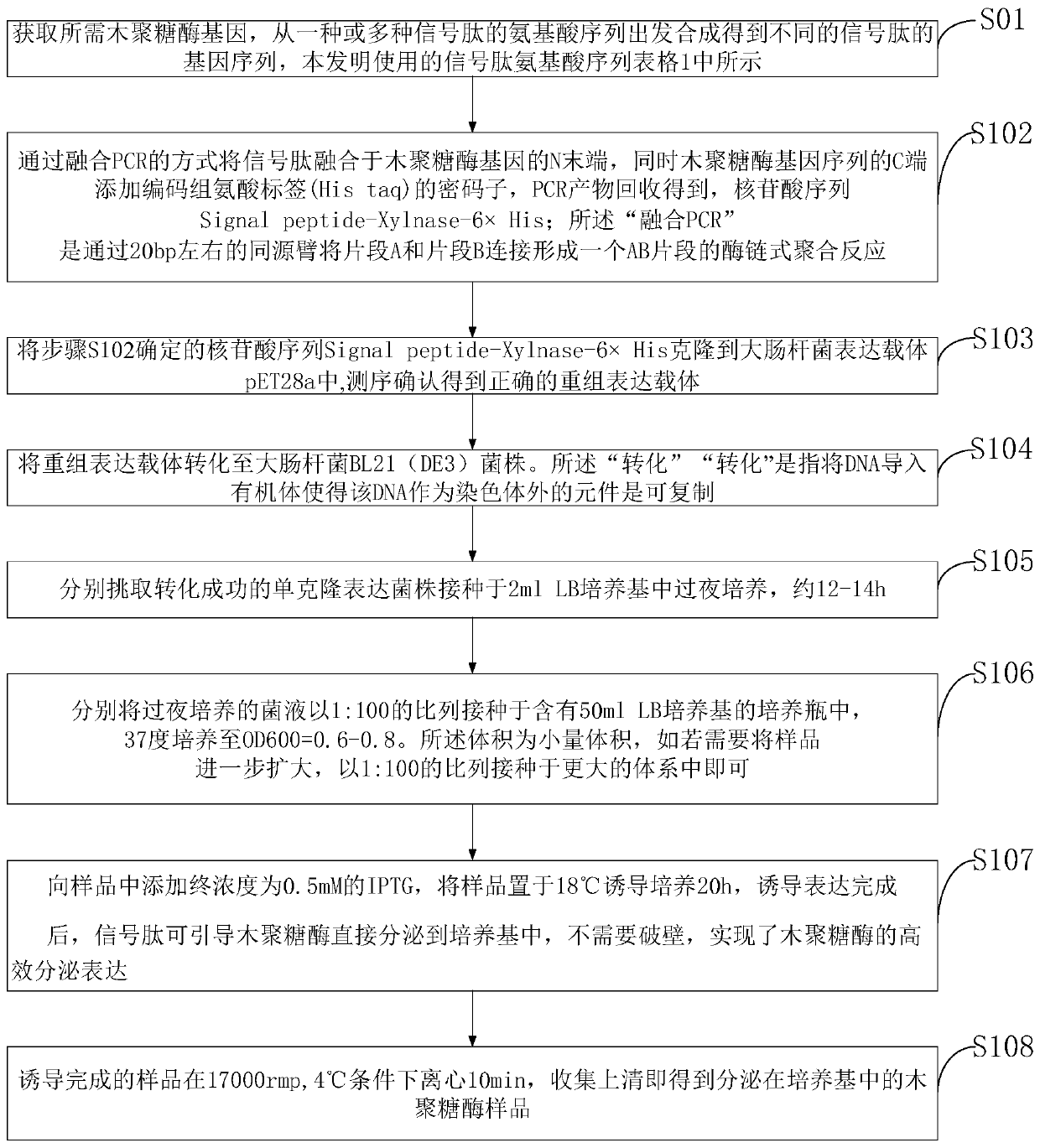
[0077] Embodiment 1: the construction of alkaline xylanase XynHB escherichia coli secretion expression vector:
[0078] 1) In the specific implementation of the present invention, the alkaline xylanase XynHB is derived from the mature form of Bacillus subtilis HBP8, and the 27-amino acid signal peptide at its N-terminal has been removed. (NCBI: AY954630.1).
[0079] 2) In the specific implementation of the present invention, the secretory expression strategy is a signal peptide that can promote extracellular secretion of the protein by fusing the N-terminus of xylanase mature XynHB.
[0080] 3) The signal peptides include well-characterized signal peptides OmpA, PelB, PhoA, and Lpp derived from the Sec pathway, signal peptides TorA and Fdog from the Tat pathway, and signal peptide FhuD derived from the Sec-Tat pathway. The amino acid sequence of the signal peptide is shown in the table below
[0081]
[0082] 4) According to the Escherichia coli codon preference table, th...
Embodiment 2
[0103] Secreted expression of alkaline xylanase XynHB in E. coli:
[0104] 1) The successfully constructed XynHB expression vectors were transformed into Escherichia coli BL21(DE3) strains, spread on kana-resistant plates and cultured overnight until single clones grew out.
[0105] 2) Pick a single clone from the plate and transfer it to 3ml LB (containing 50ug / ml kanamycin), and place it on a shaker at 37°C and 200rpm for overnight culture for 12-14h.
[0106] 3) Transplant into 50ml LB (containing 50ug / ml kanamycin) with 1% inoculum size, and continue culturing at 37°C until OD. When the 6 0 0 is 0.5-0.6, add 0.5mM IPTG at a final concentration and place on a shaker at 18°C for 20h.
[0107] 4) The induced sample was centrifuged in a high-speed refrigerated centrifuge at 17,000 rpm and 4° C. for 15 minutes, and the supernatant was collected to obtain a crude xylan enzyme sample secreted in the culture medium.
[0108] 5) Purification and identification of xylanase:
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com