Preparation method of 1-cyclopropyl-3-(2-methylthio-4-trifluoromethylphenyl)propyl-1,3-dione
A technology of trifluoromethyl phenyl and trifluoromethyl benzoic acid, applied in the field of organic synthesis, can solve problems such as high cost, unfavorable environmental protection, and many waste acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
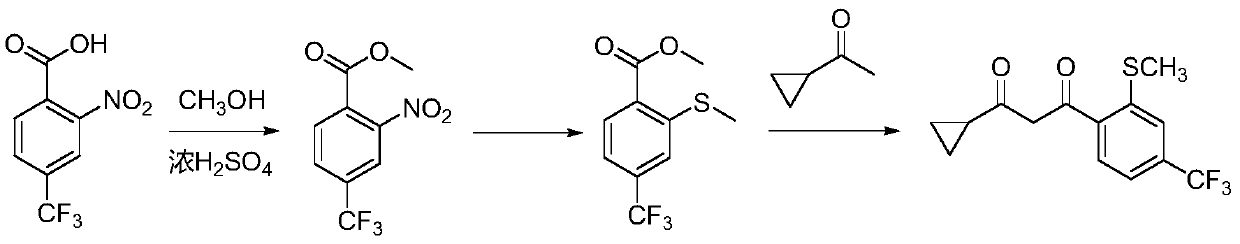
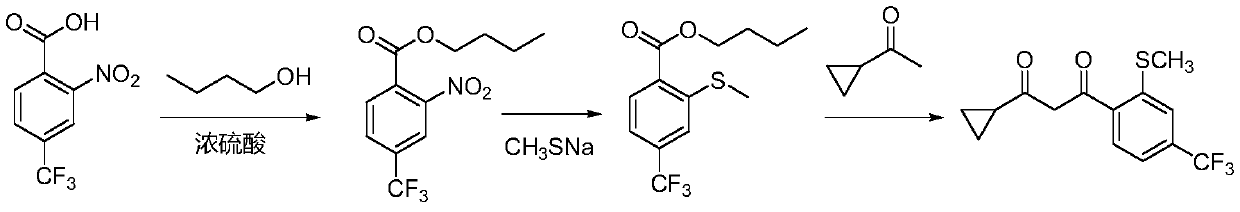
[0029] Specifically, the preparation method of 1-cyclopropyl-3-(2-methylthio-4-trifluoromethylphenyl)propane-1,3-dione includes the following steps:
[0030] (1) Mix 2-nitro-4-trifluoromethylbenzoic acid with n-butanol and concentrated sulfuric acid, and react to obtain butyl 2-nitro-4-trifluoromethylbenzoate;
[0031] (2) 2-Nitro-4-trifluoromethyl butyl benzoate is reacted with sodium methyl mercaptan to obtain butyl 2-methylthio-4-trifluoromethyl benzoate;
[0032] (3) Reaction of 2-methylthio-4-butyl trifluoromethyl benzoate with cyclopropyl ketone to obtain 1-cyclopropyl-3-(2-methylthio-4-trifluoromethylphenyl) ) Propane-1,3-dione.
[0033] The reaction formula of the method of the present invention is as follows:
[0034]
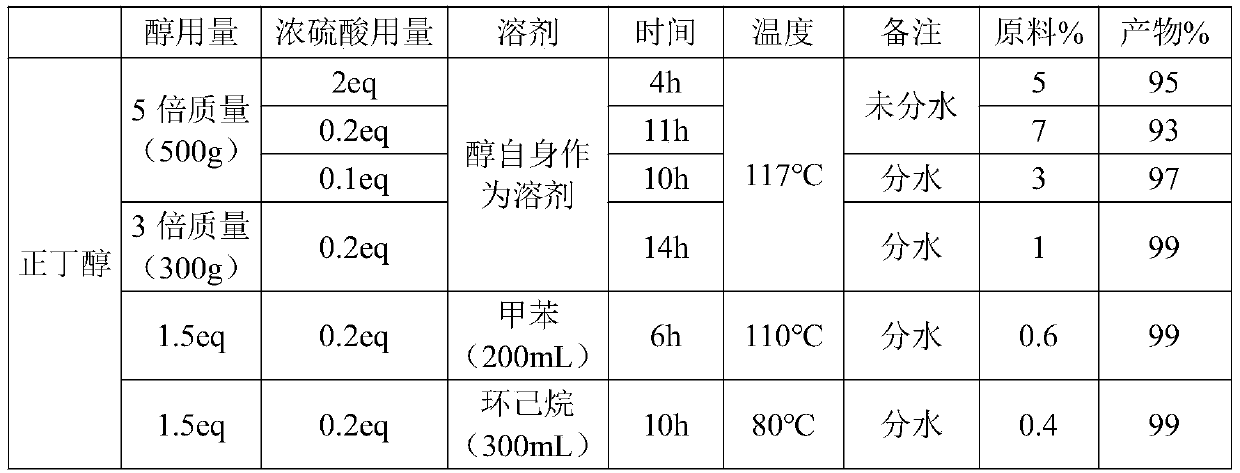
[0035] The invention selects n-butanol as the esterification raw material, which can reduce the consumption of concentrated sulfuric acid. Among them, the molar ratio of 2-nitro-4-trifluoromethyl benzoic acid to n-butanol is 1:1-16; further, in order to comple...
Embodiment 1
[0053] (1) Preparation of 2-nitro-4 butyl trifluoromethyl benzoate
[0054]
[0055] A 500mL four-neck flask was first added with 200mL of toluene, then 100g of 2-nitro-4-trifluoromethylbenzoic acid (0.425mol, 1eq), 33.1g of n-butanol (0.447mol, 1.05eq) and 4.25g of concentrated sulfuric acid ( 0.0425mol, 0.1eq), then heated to an internal temperature of 117°C to make it reflux, the water separator was separated, and the reaction was kept for 5 hours. After the reaction was kept for 5 hours, HPLC detected that there was almost no remaining 2-nitro-4-trifluoromethylbenzoic acid in the reaction solution ( 1% or less), the reaction solution is the crude product of 2-nitro-4 trifluoromethyl butyl benzoate, and the HPLC purity is 99%.
[0056] (2) Preparation of 2-methylthio-4 butyl trifluoromethyl benzoate
[0057]
[0058] Take another 1L four-neck flask, add 2.74g tetrabutylammonium bromide (0.00851mol, 0.02eq) and 164g 20% sodium methyl mercaptan (0.468mol, 1.1eq), heat to 60℃ and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com