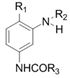
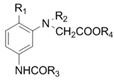
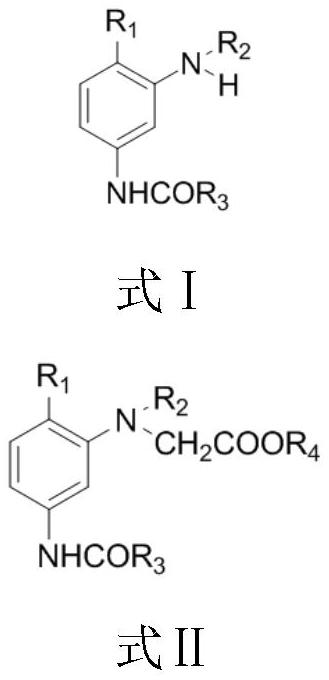
A kind of synthetic method of the dye intermediate with n-acetate group structure
The technology of a dye intermediate and a synthesis method is applied in the synthesis field of the dye intermediate, and can solve the problems of low reaction temperature, difficulty in recovery and reuse, large proportion of methyl chloroacetate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of 2-methoxy-5-acetylamino-N,N-bis(ethyl acetate)aniline
[0030]In a 250mL three-necked flask with a stirrer, a thermometer and a reflux condenser (with a water separator), add 100.0 g of ethyl chloroacetate, 36.0 g of 2-methoxy-5-acetamidoaniline, and 23.2 g of sodium carbonate. Mix evenly under stirring, heat up to 90-105°C for reflux reaction for 2.0 hours; then add 3.0g of catalyst and 0.20g of zinc powder, heat up to 105-120°C, and carry out water separation and reflux reaction for 3.0h. Samples were taken and the purity of the target product was tested by high pressure liquid chromatography. After recovering ethyl chloroacetate by distillation under reduced pressure, add acetic acid to obtain an acetic acid solution containing 2-methoxy-5-acetylamino-N,N-bis(ethyl acetate)aniline, measure the coupling value, and calculate the yield . The results are shown in Table 1.
[0031] Table 1: Purity and Yield List of Synthesized Products Using Four Kinds of ...
Embodiment 2
[0038] Synthesis of m-Acetamido-N,N-bis(methylacetate)aniline
[0039] In a 250mL three-neck flask with a stirrer, a thermometer and a reflux condenser (with a water separator), add 108.5g of methyl chloroacetate, 30.0g of m-acetamidoaniline, and 23.2g of sodium carbonate, mix well under stirring, and heat up to Reflux reaction at 90-100°C for 2.0h; then add 2.0g of sodium bromide and 0.30g of zinc powder, raise the temperature to 105-110°C, and carry out water-dividing reflux reaction for 2.0h. Sampling was carried out and detected by high-pressure liquid chromatography. The purity of the main component m-acetamido-N,N-di(acetoxymethyl)aniline was 99.4%. After recovery of methyl chloroacetate by vacuum distillation, acetic acid was added to obtain an acetic acid solution containing m-acetamido-N,N-di(acetoxymethyl)aniline. The yield was 98.6% by measuring the coupling value.
Embodiment 3
[0047] Synthesis of m-propionylamino-N,N-bis(ethyl acetate)aniline
[0048] In a 250mL three-necked flask equipped with a stirrer, a thermometer, and a reflux condenser (with a water trap), add 110.0 g of ethyl chloroacetate, add 33.0 g of m-propionamidoaniline, and 25.0 g of sodium carbonate under stirring. well mixed. Raise the temperature to 100-110°C, and reflux for 1.0h. Add 2.5g of sodium bromide and 0.25g of zinc powder, and reflux at 110-125°C for 3.0h. Sampling and detection by high-pressure liquid chromatography showed that the target product had a purity of 99.2%. After the ethyl chloroacetate was recovered by distillation under reduced pressure, acetic acid was added to obtain the acetic acid solution of m-propionylamino-N,N-bis(ethyl acetate)aniline, with a yield of 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com