Process for preparing 3-amino-2-naphthoic acid compound
A preparation process, a technology of naphthoic acid, applied in the preparation of carbamic acid derivatives, the preparation of organic compounds, the preparation of cyanide reactions, etc., can solve the problems of high preparation cost, poor applicability, unstable reaction, etc., and reduce the use of special equipment. The effect of using, wide application and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
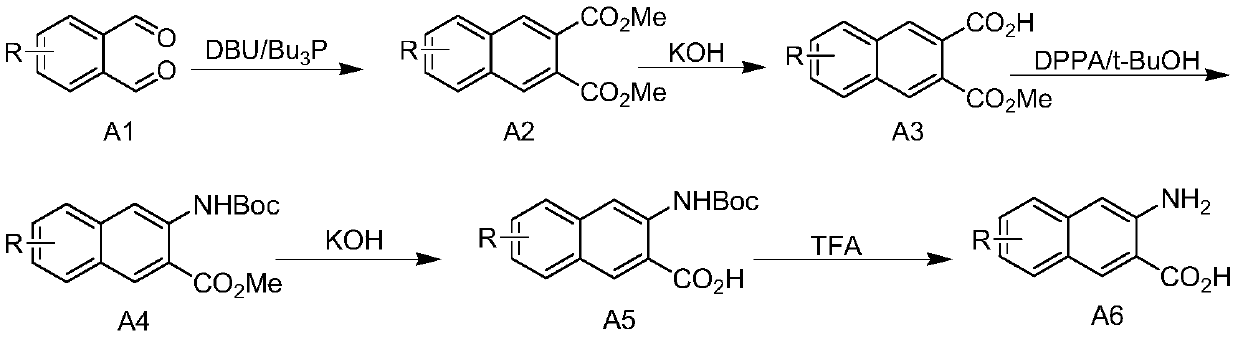
[0030] A kind of preparation technology of 3-amino-2-naphthoic acid compounds:
[0031] (1) 15.84g of dimethyl maleate was dissolved in 300mL of dichloromethane, and the resulting solution was cooled with an ice bath, and 24.24g of tri-n-butylphosphine was added dropwise to the low-temperature solution. Rise to room temperature, and continue to stir the reaction for more than 0.5h. Dissolve 13.4g of o-phthalaldehyde in 200mL of dichloromethane solution, and slowly add the mixture obtained above under cooling in an ice bath, then add 1.52g of DBU dropwise, and stir the resulting mixture at room temperature for 3h. Quench the reaction with 400mL ice water, separate the organic phase, extract the aqueous phase twice with 200mL dichloromethane, combine the organic phases, wash with 150mL saturated brine, dry the organic phase with anhydrous sodium sulfate for 2h, concentrate under reduced pressure, and the product column After separation by chromatography, 22.3 g of dimethyl 2,3-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com