7-nitro-1,2,3-benzooxadiazole derivative and synthesis method and application thereof
A technology of benzoxadiazole and synthesis method, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve problems such as mercury pollution, human health hazards, protein and enzyme dysfunction, etc., and achieve simple and convenient detection methods. , The operation is simple and convenient, and the results are clear and identifiable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis and characterization of embodiment 1NBD derivatives
[0037] In the first step, 0.1g (0.5mmol) NBD-Cl and 0.13g (3.5mmol) anhydrous piperazine were dissolved in 7ml 2-methoxyethanol, in N 2 Heated under protection and refluxed for 6h. After the reaction, suction filtered and separated by column chromatography to obtain compound 1. 1HNMR (DMSO-d6) δ: 8.47 (d, 1H, ArH), 6.66 (d, 1H, ArH), 4.08 (m ,4H,CH 2 ),2.92(m,4H,CH 2 ).
[0038] In the second step, 75 mg of anhydrous K 2 CO 3 Add 0.175g (1mmol) of 2,6-bis(chloromethyl)pyridine in acetone solution, heat to reflux for 1h, dropwise add the acetone solution containing 8-hydroxyquinoline (0.036g, 0.25mmol) in the mixture, and react The mixture was heated to reflux for 6 h, filtered, and the solvent was removed in vacuo to obtain a white solid, and then the crude product was chromatographed on a silica gel column to obtain compound 2. 1H NMR (DMSO-d6) δ: 9.07 (s, 1H, ArH), 8.23 (d,1H,ArH),7.74(t,1H,ArH),7.6...
Embodiment 2
[0040] Embodiment 2NBD derivatives are used for Hg 2+ Fluorescence spectrometry
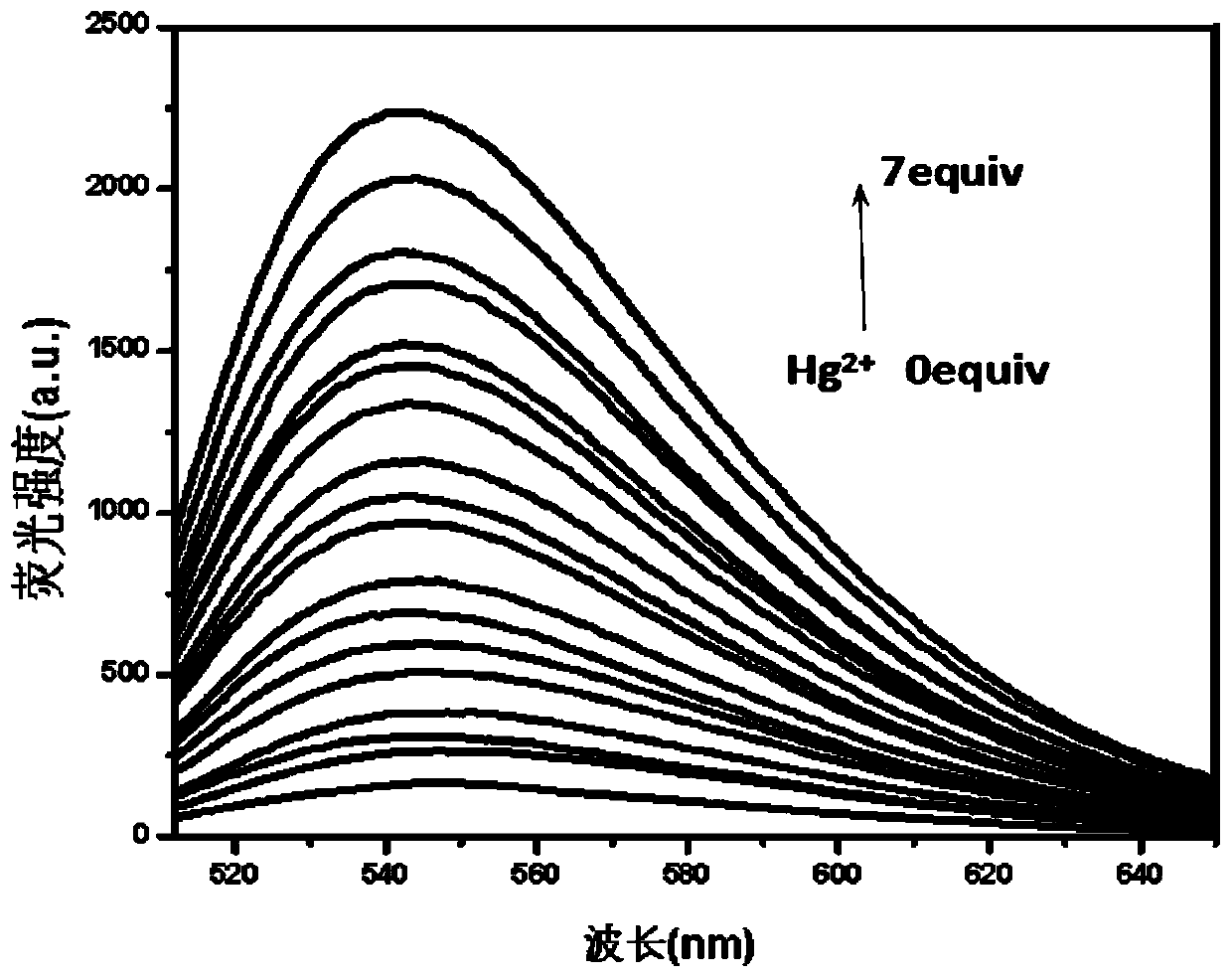
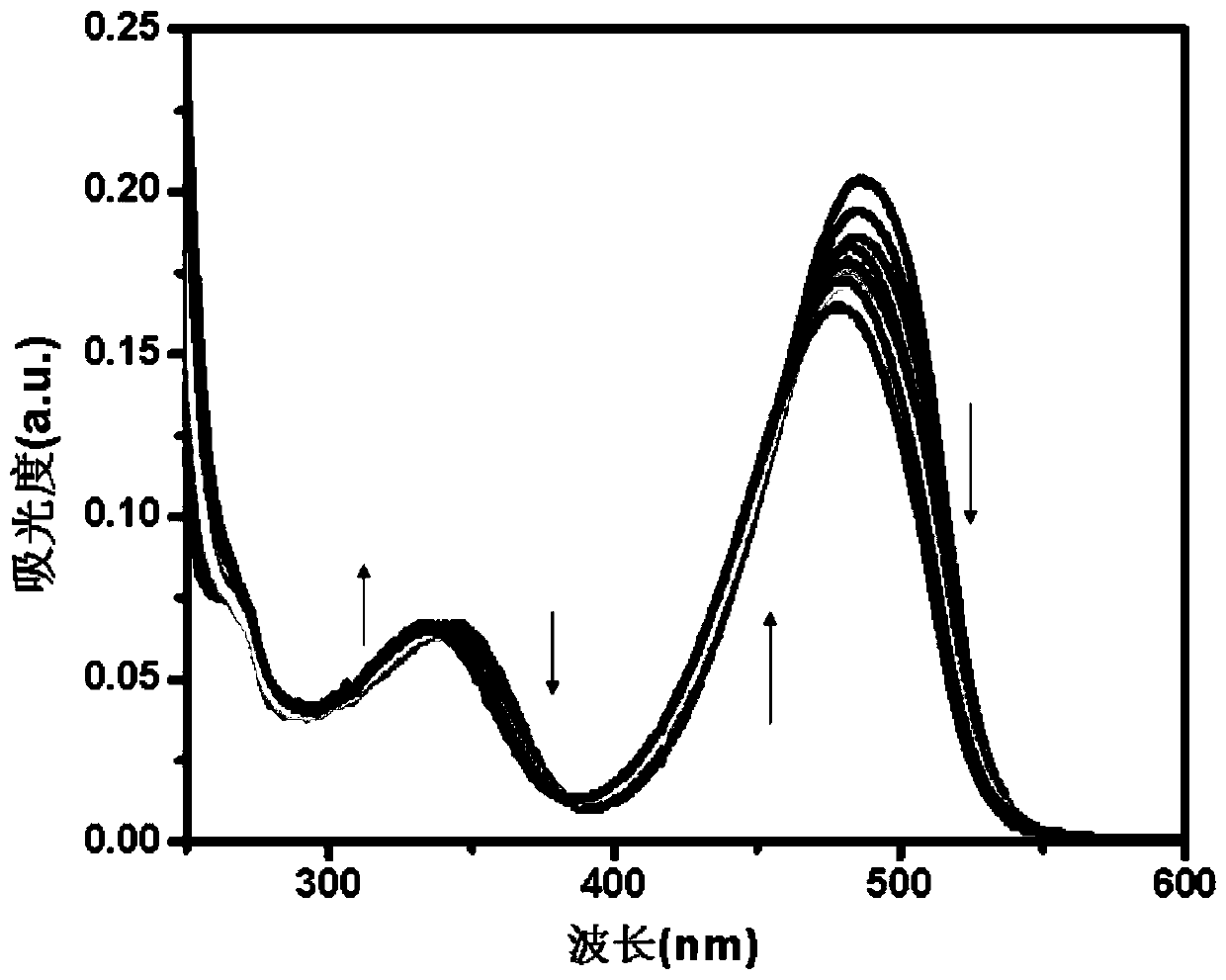
[0041] Prepare 1mM NBD derivative stock solution in DMSO and 0.01M Hg in distilled water 2+ Solution, and configure HEPES buffer solution with pH = 7.4 and concentration of 0.025M; take 20 μL of NBD derivative stock solution and add it to a clean colorimetric tube, and add different volumes of Hg 2+ (2μL, 4μL, 6μL, 8μL, 10μL, 12μL, 14μL), 0.5mL HEPES buffer, dilute to 5mL with secondary water, shake well, take 2.5mL into a clean cuvette, and put it on the fluorescence spectrophotometer detection, with Hg 2+ The addition of , the solution gradually changed from orange to yellow, and the fluorescence intensity at 545 nm gradually increased. For the fluorescence spectrum, see figure 1 .
Embodiment 3
[0042] Embodiment 3 NBD derivative measures Hg 2+ linear relationship
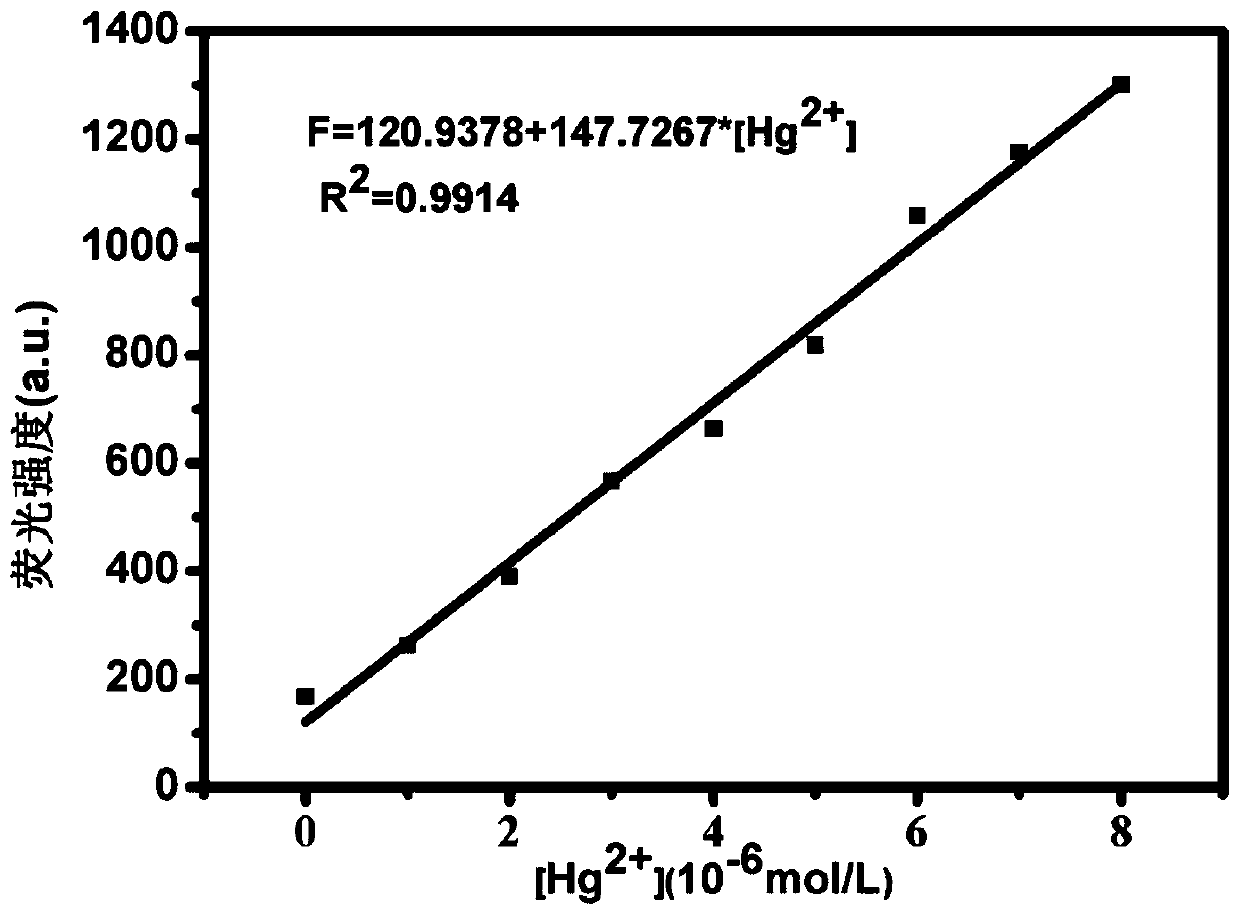
[0043] Take 20 μL of NBD derivative stock solution and add it to a clean colorimetric tube, and add different volumes of Hg 2+ (2μL, 4μL, 6μL, 8μL, 10μL, 12μL, 14μL), 0.5mL HEPES buffer, dilute to 5mL with secondary water, shake well, take 2.5mL into a clean cuvette, and put it on the fluorescence spectrophotometer detection, with Hg 2+ Adding, the solution gradually changes from orange yellow to yellow, the fluorescence intensity at 545nm gradually increases, and the fluorescence intensity of the system at 545nm I 545nm and [Hg 2+ ]Concentration in 0-8×10 -6 A good linear relationship is exhibited in the range of M (R 2 =0.9914), in Hg 2+ The concentration is the abscissa, and the fluorescence intensity I 545nm Plot the ordinate to get Hg 2+ Linear equation of concentration and fluorescence intensity: F=120.9378+147.7267[Hg 2+ ], [Hg 2+ ] has a unit of 10 -6 mol / L; working linear diagram see ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com