An organic small molecule high-efficiency room temperature phosphorescent material based on aroimide and its preparation and application
A reaction and substituent technology, applied in the field of organic luminescence and its anti-counterfeiting, can solve the problems of difficult mass production, long synthesis steps, and few molecular types, etc., and achieve the effect of cheap raw materials, simple synthesis method, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The reaction equation is as follows:
[0037]
[0038] The specific steps of the reaction are as follows:
[0039] A mixture of compound formula A-1 and compound formula B-1 in glacial acetic acid was refluxed for 4 hours. The solid was separated from the mixture (the reaction liquid was cooled to room temperature, and the precipitate was filtered), and the product formula I-1 was obtained.
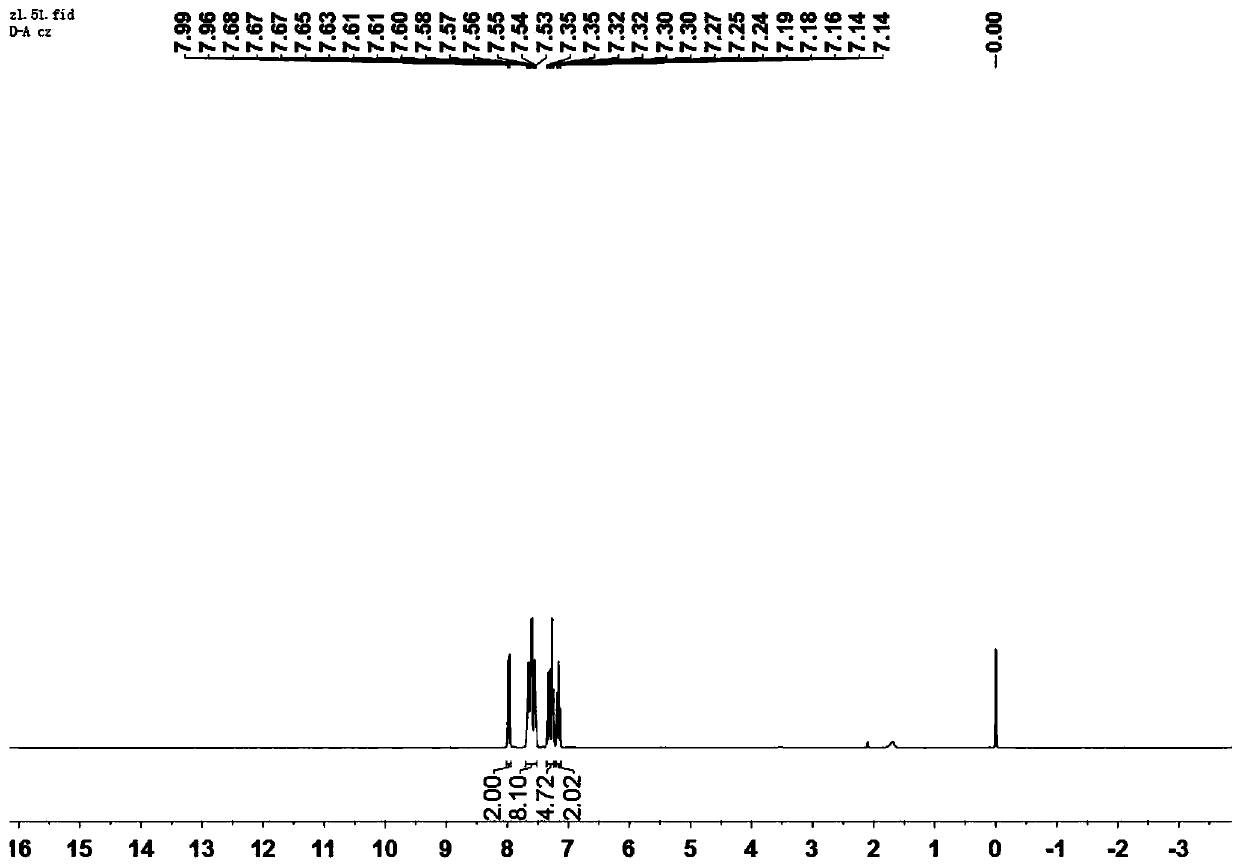
[0040] The H NMR spectrum of the compound shown in formula Ⅰ-1 is as follows: figure 1 As shown, the characterization data are as follows:
[0041] 1 H NMR (300MHz, Chloroform-d) δ7.98 (d, J=7.7Hz, 2H), 7.70–7.51 (m, 8H), 7.36–7.23 (m, 4H), 7.20–7.12 (m, 2H), HR-MS(APCI)m / z calcd for C 26 h 16 N 2 o 2 [M+H] + 389.12, found 389.13.
[0042] From the above detection results, it can be seen that the structure of the compound represented by formula I-1 is correct.
Embodiment 2
[0044] The reaction equation is as follows:
[0045]
[0046] The specific steps of the reaction are as follows:
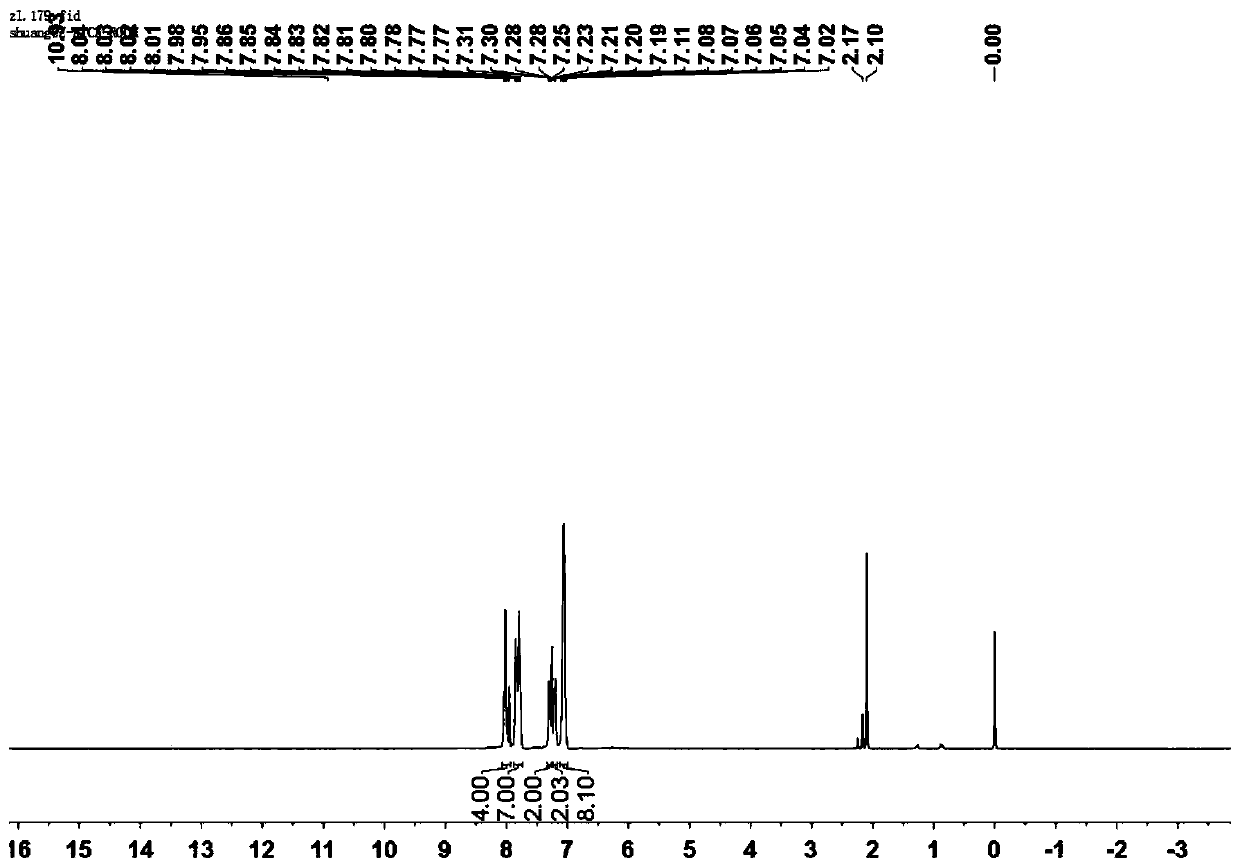
[0047] A mixture of compound formula A-1 and compound formula B-1 in glacial acetic acid was refluxed for 4 hours. The solid was separated from the mixture (the reaction liquid was cooled to room temperature, and the precipitate was filtered), and the product formula I-2 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com