Antibody for inhibiting replication of PRRS virus, expression vector and therapeutic agent
A virus replication and expression vector technology, applied in the field of genetic engineering, can solve problems such as limited protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Prokaryotic expression of antibody therapeutic agent:
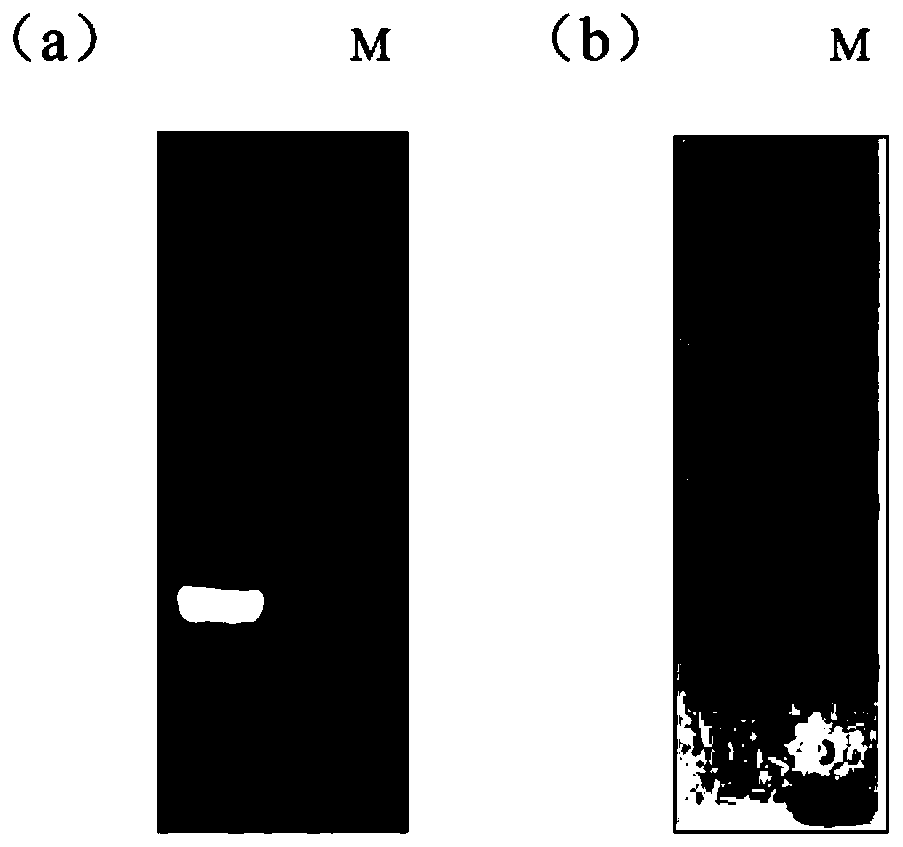
[0036] Through two rounds of PCR (primers and templates are as follows), the gene encoding the antibody therapeutic agent is amplified. NdeI and XhoI (purchased from NEB) were used to digest 20 μg of the pET21b prokaryotic expression vector and the product recovered by the second round of PCR, and then the two fragments were ligated at 16° C. overnight with T4 DNA ligase. Take 10 μl of the ligation product and transform it into 50 μl of expression competent cells BL21(DE3), and spread it on LB after activation AMP+ Agar plate, cultivate overnight at 37°C, pick a single colony in LB AMP+ Cultivate in liquid medium at 37°C and 220rpm for 12h, and perform sequencing analysis on the bacterial liquid identified as positive by bacterial liquid PCR. Bacteria with positive sequencing results were inoculated in LB AMP+ Cultivate in culture medium. After the bacteria grow to the logarithmic phase, use 1mM IPTG t...
Embodiment 2
[0048] Example 2 Enzyme-linked immunoassay detection of interaction between antibody therapeutic agent and Nsp9
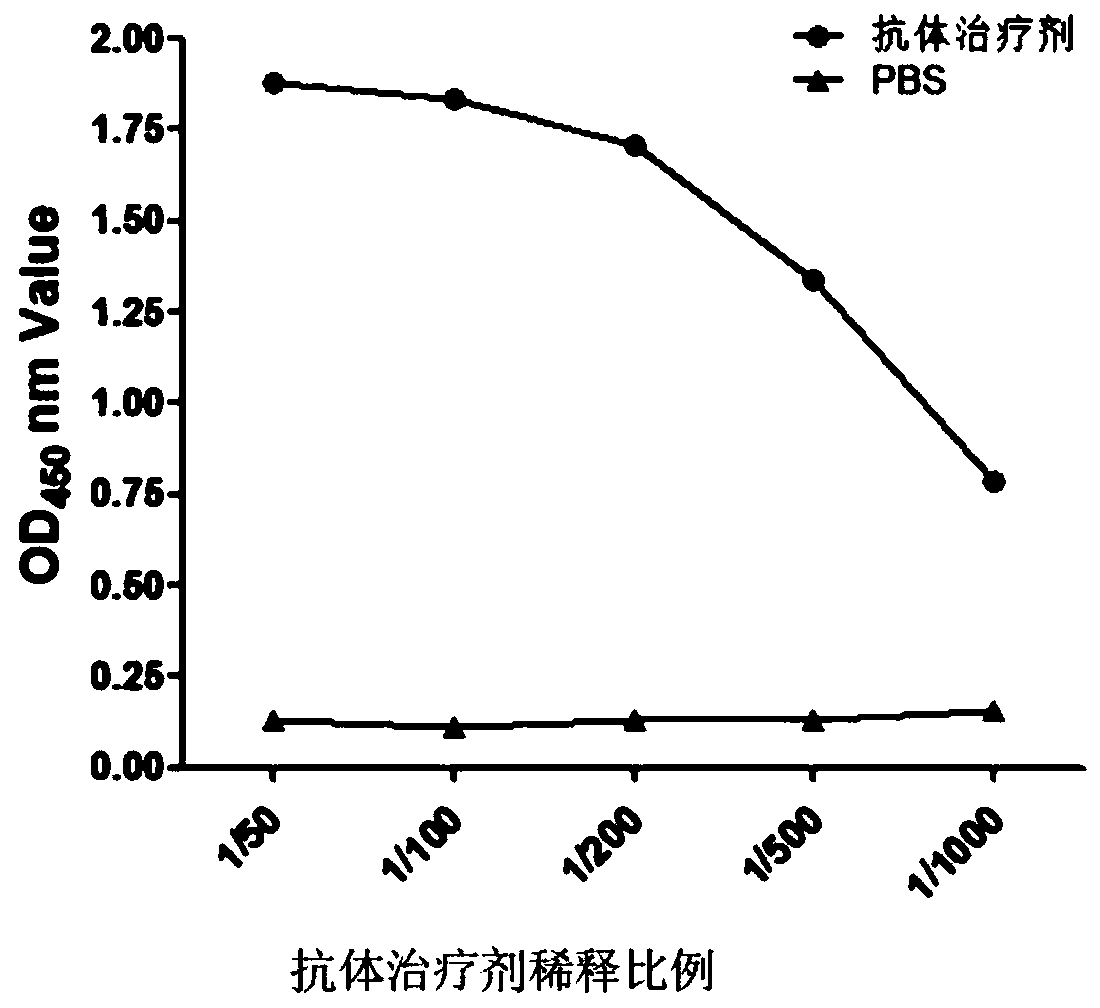
[0049] First, Nsp9 was coated with PBS (0.01M, pH7.2) on the ELISA plate, 400ng per well, overnight at 4°C. After the ELISA plate was washed 3 times with PBS-T (PBS containing 0.5% Tween-20 by volume), it was blocked for 1 hour with a blocking solution (2.5% skimmed milk powder was dissolved in PBS-T, 200 μl / well); washed 3 times, Add antibody therapeutics with different dilution ratios to each well for reaction for 1 hour; wash 3 times, add mouse anti-His monoclonal antibody to each well and react for 1 hour; wash 3 times, add horseradish peroxidase (HRP)-labeled antibody to each well Goat anti-mouse IgG was reacted for 1 hour, washed 3 times; 100 μl of HRP substrate (TMB) was added to each well for 15 minutes, and 50 μl of 1M sulfuric acid was added to each well to stop the reaction, and the OD value of each well was read at 450 nm with a microplate reader. See ...
Embodiment 3
[0050] Example 3 Antibody Therapeutics Enter Cells
[0051] After recovery of PAMs cells, 1×10 per well 6 The density was laid in a 24-well cell culture plate, cultured for 4 hours, the old medium was discarded, and new serum-free RPMI 1640 medium was added, and the antibody therapeutic agent was added to the cell culture medium of each well at a final concentration of 5 μM, and after 5 hours of culture, Intracellular antibody therapeutics were detected using indirect immunofluorescence assays. The specific steps are as follows: discard the supernatant, wash the cells three times with PBS, add 4% paraformaldehyde, fix the cells at room temperature for 15 minutes, and permeate the membrane with 0.25% Triton X-100 at room temperature for 10 minutes. Wash 3 times with PBS, add mouse His monoclonal antibody after blocking with 1% BSA, and incubate at room temperature for 1 h. Wash 3 times with PBS, add Alexa 488-labeled goat anti-mouse fluorescent secondary antibody, placed at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com