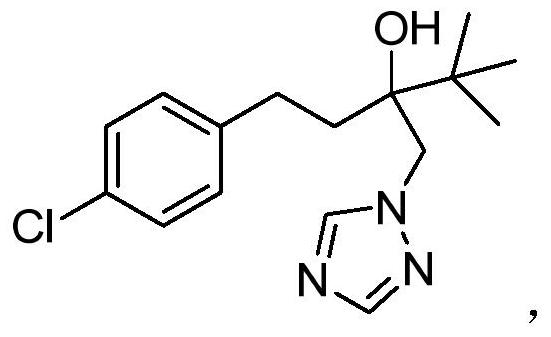
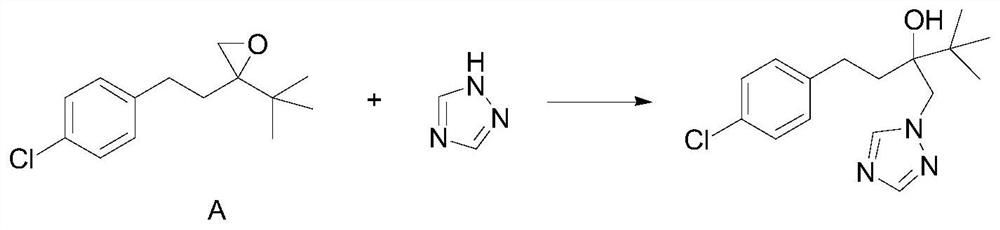
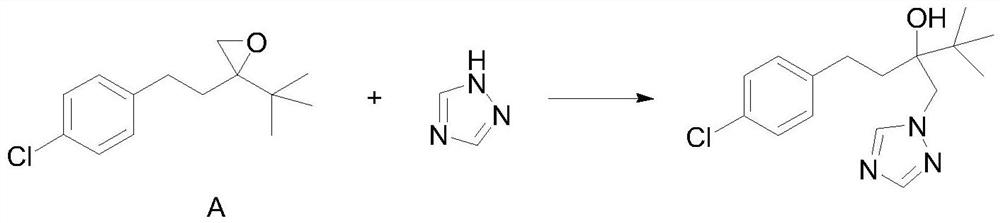
A kind of cleaning preparation method of tebuconazole
A tebuconazole and clean technology, applied in the field of clean preparation of tebuconazole, can solve problems such as low reaction yield, inability to realize clean preparation of tebuconazole, difficult recovery of reaction solvent, etc., and achieve simple and easy post-processing, high The effect of industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] In a preferred embodiment, the cleaning preparation method of tebuconazole comprises the following specific steps:
[0029] S1: Add 1,2,4-triazole, potassium hydroxide, diethylene glycol monomethyl ether into a reaction vessel, after heating, slowly add the reaction material A dropwise, and then keep warm for reaction;
[0030] S2: After the reaction is complete, let stand to cool, filter with suction, take the filter cake, wash the filter cake with n-hexane, and dry to obtain the target product tebuconazole.
[0031] In a further preferred embodiment, the molar amount of the potassium hydroxide is 0.1-1.5 times the molar amount of the reaction raw material A.
[0032] In a further preferred embodiment, the mass of the diethylene glycol monomethyl ether is 0.5-10.0 times the mass of the reaction raw material A.
[0033] In a further preferred embodiment, the duration of slowly adding the reaction material A dropwise is 0.5-5 hours.
[0034] In a further preferred embo...
Embodiment 1
[0044] Add 20.5g of 1,2,4-triazole, 100.0g of diethylene glycol monomethyl ether and 6.5g of potassium hydroxide to a 500ml three-necked flask, heat in an oil bath, and control the internal temperature at 100-105°C; Then, slowly add 60.0 g of the reaction material A dropwise, and the dropping time is 2 hours. After the drop is completed, keep stirring at 100-110° C. for 10 hours. TLC monitors that the reaction of the reaction material A is complete. Then, the reaction solution was allowed to stand and cooled to room temperature, kept in an ice-water bath for 1 h, suction filtered, and the mother liquor collected by suction filtration was used mechanically, and the filter cake was taken, rinsed with 50ml of n-hexane, and vacuum-dried to obtain 67.9 g of tebuconazole product quality, The purity is 98.0%, and the total yield is 91.0%. The tebuconazole product is characterized as follows: GCMS (M+): 307.2[6], 274.1[6], 250.1[100], 207.1[11], 163.1[14], 125.1[100], 103.1[12], 83.1[...
Embodiment 2
[0046]Add 21.0g of 1,2,4-triazole, 150.0g of diethylene glycol monomethyl ether and 7.0g of potassium hydroxide to a 500ml three-necked flask, heat in an oil bath, and control the internal temperature at 100-110°C; Then, slowly add 60.0 g of the reaction material A dropwise, and the dripping duration is 1 hour. After the drop is completed, keep stirring at 100-120° C. for 10 hours. TLC monitors that the reaction material A is completely reacted. Then, the reaction solution was allowed to stand and cooled to room temperature, kept warm in an ice-water bath for 1 hour, suction filtered, and the mother liquor collected by suction filtration was used mechanically, and the filter cake was taken, rinsed with a mixed solvent of 50ml of n-hexane and petroleum ether, and vacuum-dried to obtain tebuconazole The quality of the alcohol product is 68.6g, the purity is 98.2%, and the total yield is 92.1%. The tebuconazole product characterization data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com