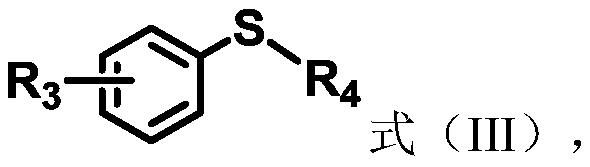
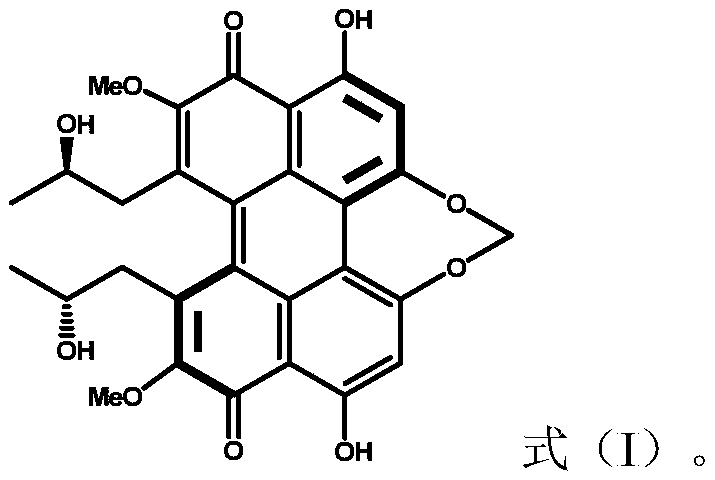
Method for photocatalytic synthesis of sulfur sulfone compounds
A technology of thioether compounds and compounds, which is applied in the field of catalysis, can solve the problems of difficult preparation of catalysts, harsh oxidation conditions, and poor reaction selectivity, and achieve the effects of low cost, high reaction efficiency, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 cercosporin catalyst catalyzes the synthesis of benzyl phenyl sulfone
[0031] Cercosporin (0.005 mmol), benzyl phenyl sulfide (0.5 mmol), and 2 mL of methanol were sequentially added into a 10 mL reaction tube, then protected by oxygen, irradiated with 15W white light, and reacted at room temperature 25 degrees Celsius for 24 hours. The reaction solution was evaporated to dryness by rotary evaporation, and then quickly separated on a 300-500 mesh thin-layer silica gel plate. The eluent used was ethyl acetate / petroleum ether (v:v=1:5) to obtain benzylphenyl sulfone, The yield was 90%.
Embodiment 2
[0032] Embodiment 2 cercosporin catalyst catalyzes the synthesis of benzyl benzyl sulfone
[0033] Cercosporin (0.005 mmol), benzyl benzyl sulfide (0.5 mmol), and 2 mL of methanol were sequentially added into a 10 mL reaction tube, then protected by oxygen, irradiated with 15W white light, and reacted at room temperature 25 degrees Celsius for 24 hours. The reaction solution was evaporated to dryness by rotary evaporation, and then quickly separated with a 300-500 mesh thin-layer silica gel plate. The eluent used was ethyl acetate / petroleum ether (v:v=1:5) to obtain benzylbenzylsulfone, The yield was 95%.
Embodiment 3
[0034] Embodiment 3 cercosporin catalyst catalyzes the synthesis of phenylmethyl sulfone
[0035] Add cercosporin (0.005mmol), phenylmethyl sulfide (0.5mmol) and 2mL methanol successively into a 10mL reaction tube, then protect with oxygen, irradiate with 15W white light, and react at room temperature 25°C for 24h. The reaction solution was evaporated to dryness by rotary evaporation, and then quickly separated on a 300-500 mesh thin-layer silica gel plate. The eluent used was ethyl acetate / petroleum ether (v:v=1:5) to obtain phenylmethyl sulfone, The yield was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com