Injectable cartilage repairing hydrogel and preparation method thereof
A cartilage repair and hydrogel technology, applied in medical science, prosthesis, etc., can solve the problem of low mechanical strength of gelatin, and achieve the effect of good injectability and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] An injectable hydrogel for cartilage repair, comprising the following components in mass percentage: 1% modified hyaluronic acid, 10% modified gelatin, 1% platelet-rich plasma, 1% ultraviolet photoinitiator, and the balance is water.
[0033] The preparation method of the present embodiment hydrogel material comprises the following steps:
[0034] (1) Preparation of modified hyaluronic acid
[0035] Weigh 5g of hyaluronic acid, add 100mL of ionized water, stir until completely dissolved at room temperature, add 25g of methacrylic anhydride, adjust the pH to 8 with 1mol / L NaOH, stir and react in an ice-water bath overnight, and then load 12-14KDa In the dialysis bag, dialyze with pure water for 3 days, then freeze-dry to obtain modified hyaluronic acid, and store it at room temperature for later use;
[0036] (2) Preparation of modified gelatin
[0037] Weigh 20g of gelatin, add 100mL of deionized water, stir in a water bath at 50°C until completely dissolved, add 12g ...
Embodiment 2
[0045] An injectable hydrogel for cartilage repair, comprising the following components in mass percentage: 1% modified hyaluronic acid, 10% modified gelatin, 1% platelet-rich plasma, 2% ultraviolet photoinitiator, and the balance is water.
[0046] The preparation method of the hydrogel material in this example is the same as that in Example 1, wherein, in step (4), the modified hyaluronic acid and the modified gelatin are prepared into 2% and 20% solutions respectively, and then mixed and stirred evenly in equal volumes; Then 1% of platelet-rich plasma and 2% of ultraviolet photoinitiator were added respectively.
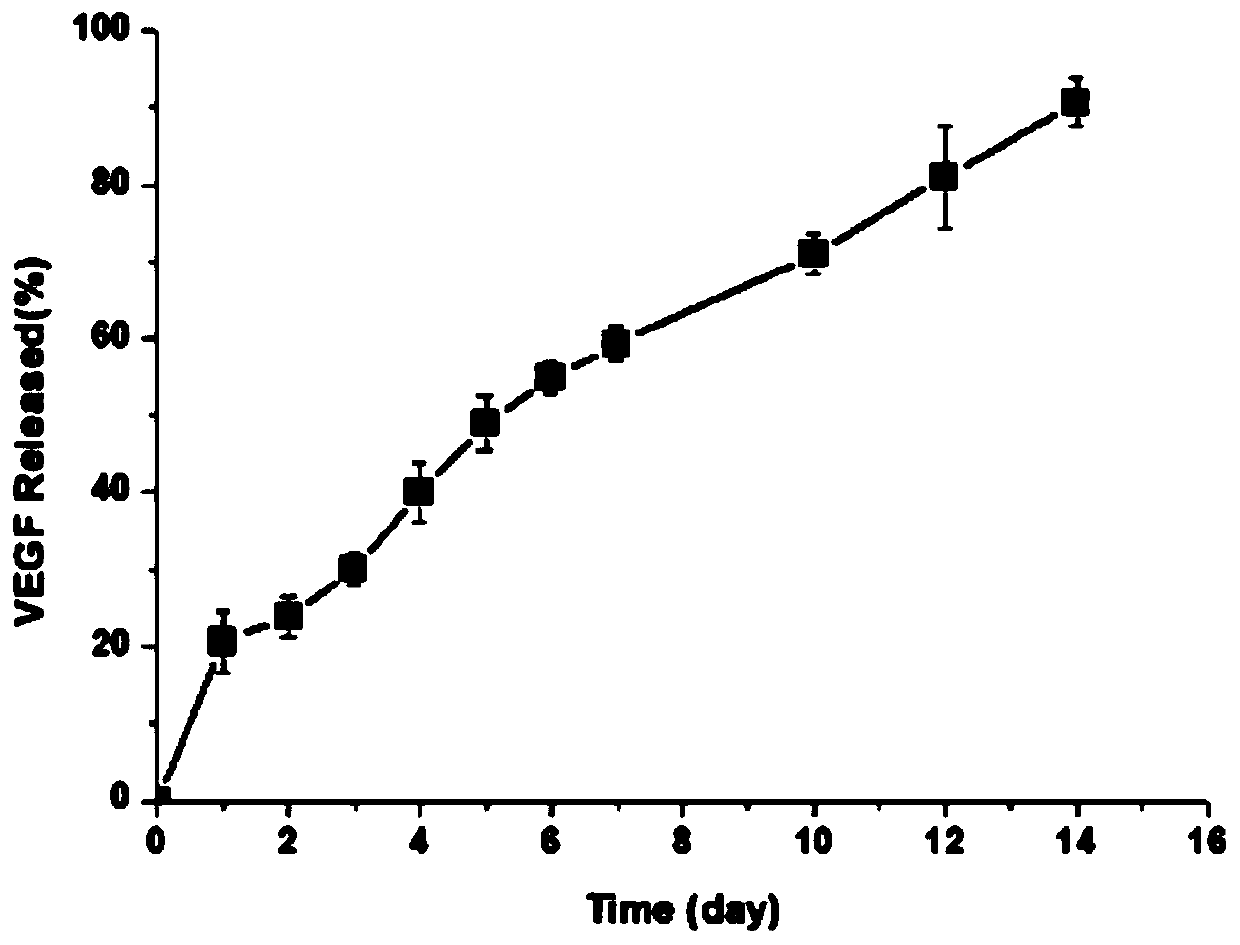
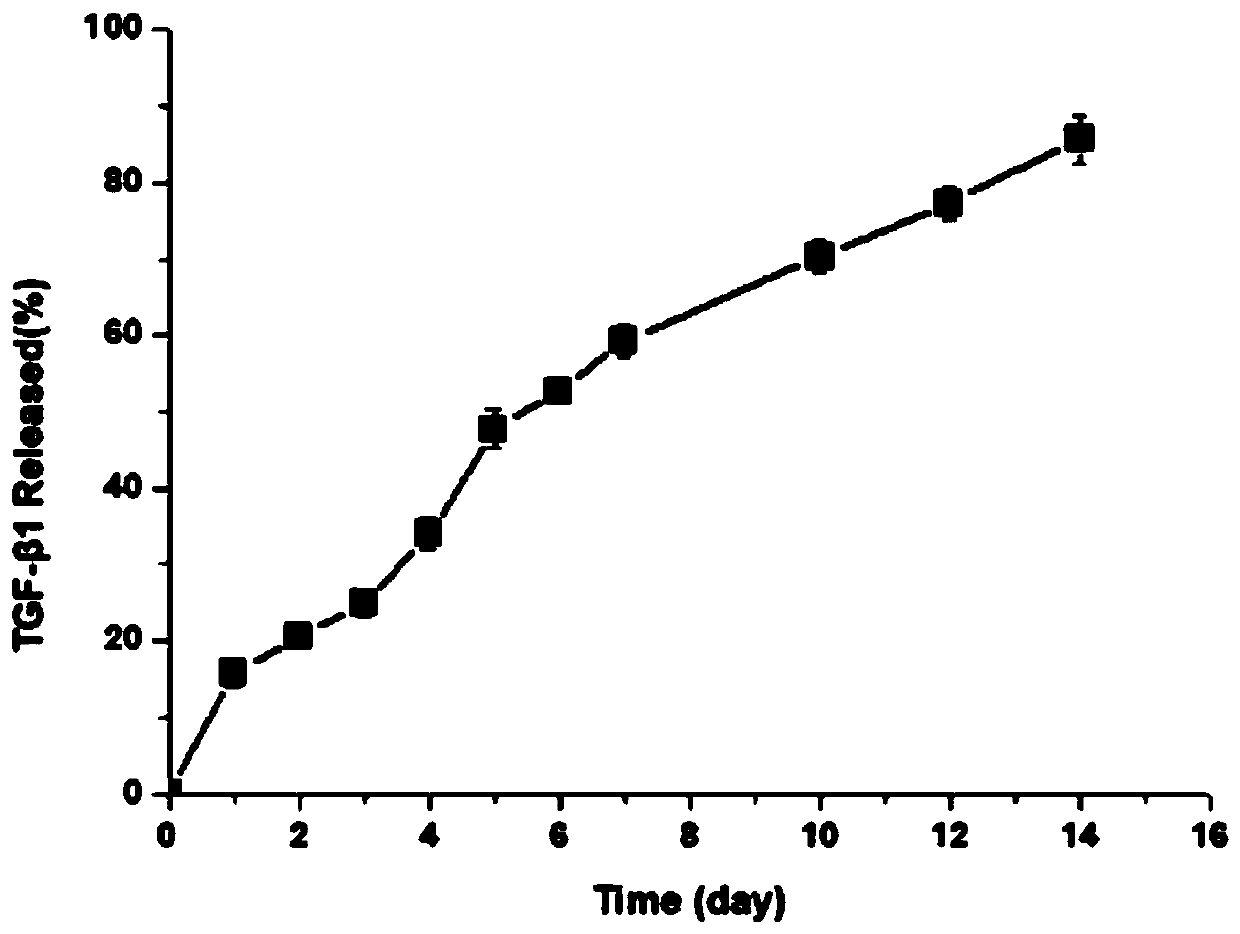
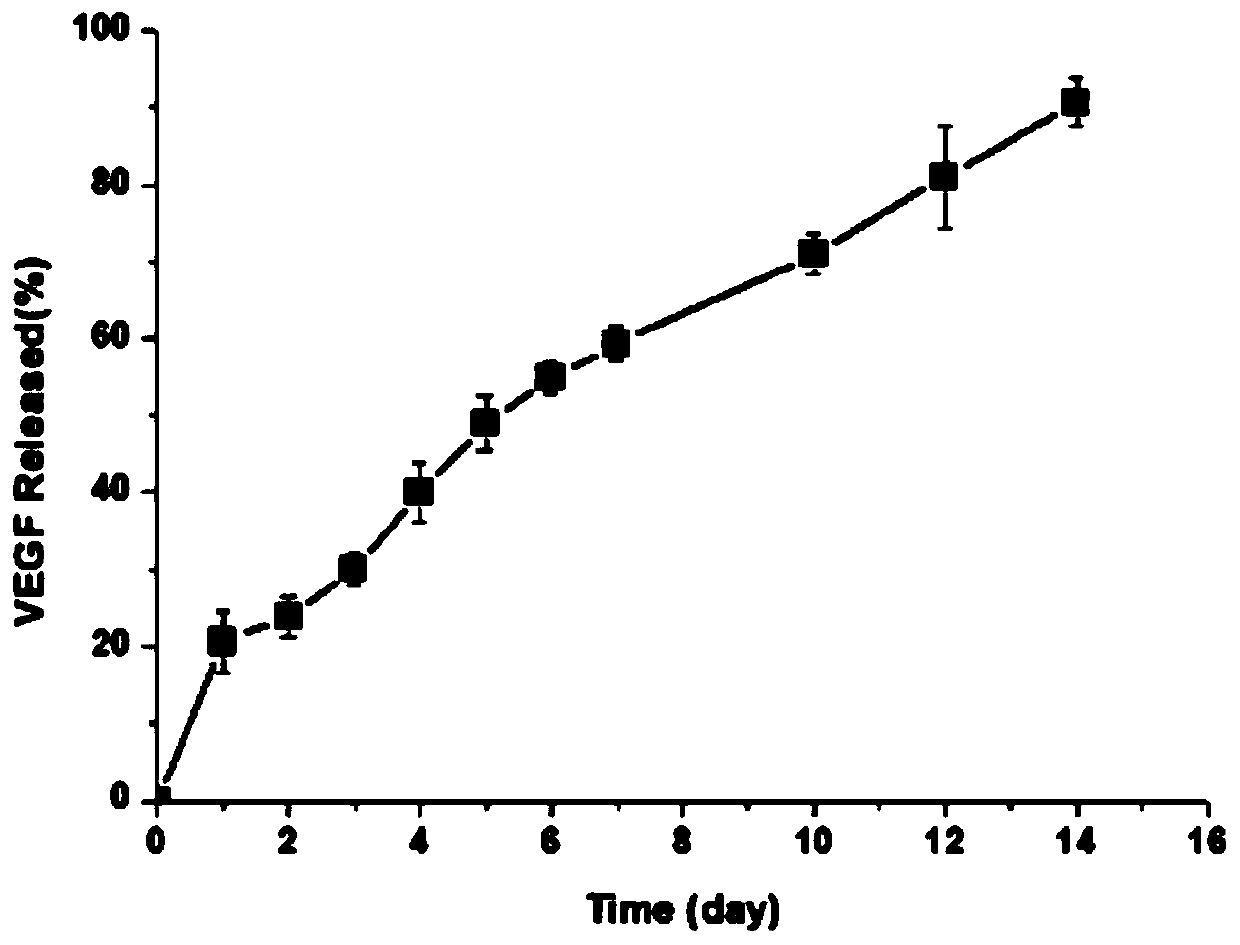
[0047] Within two weeks, the sustained release of VEGF in the gel material of this embodiment can reach about 90%; the sustained release of TGF-β1 within two weeks is close to 85%, indicating that the hydrogel material loaded with platelet-rich plasma in this embodiment can achieve both. slow release of growth factors.
Embodiment 3
[0049] An injectable hydrogel for cartilage repair, comprising the following components in mass percentage: 1% modified hyaluronic acid, 7.5% modified gelatin, 1% platelet-rich plasma, 1% ultraviolet photoinitiator, and the balance is water.
[0050] The preparation method of the hydrogel material in this example is the same as that in Example 1, wherein, in step (4), the modified hyaluronic acid and the modified gelatin are respectively configured into 2% and 15% solutions, and then mixed and stirred evenly in equal volumes; Then 1% platelet-rich plasma and 1% ultraviolet photoinitiator were added respectively.
[0051] Within two weeks, the sustained release of VEGF in the gel material of this embodiment can reach about 90%; the sustained release of TGF-β1 within two weeks is close to 85%, indicating that the hydrogel material loaded with platelet-rich plasma in this embodiment can achieve both. slow release of growth factors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com