Anti-CII chimeric antigen receptor coding gene, slow virus plasmid, Treg immune cell, and applications thereof
A technology of chimeric antigen receptors and coding genes, applied in blood/immune system cells, receptors/cell surface antigens/cell surface determinants, anti-animal/human immunoglobulins, etc., can solve the problem of no preparation method To achieve the effect of terminating the cytokine storm, ensuring the safety of clinical use, and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Anti-CII chimeric antigen receptor coding gene, including Linker nucleic acid artificial sequence, CII single-chain antibody nucleic acid artificial sequence, CD8 hinge region nucleic acid artificial sequence, CD8 transmembrane region nucleic acid artificial sequence, 2B4 co-stimulatory region nucleic acid artificial sequence, CD3ζ signal The nucleic acid artificial sequence of the conducting region, the artificial nucleic acid sequence of the T2A self-cleaving region, the artificial nucleic acid sequence of CTLA4 and the artificial nucleic acid sequence of the RQR8 molecular switch region.
[0038] Such as figure 1As shown, the anti-CII chimeric antigen receptor coding gene of the present embodiment includes the Leader nucleic acid artificial sequence (SEQ ID NO.2) connected sequentially, the CII binding region nucleic acid artificial sequence (SEQ ID NO.3), and the CD8Hinge region nucleic acid artificial sequence. sequence (SEQ ID NO.4), CD8 transmembrane region nucle...
Embodiment 2
[0040] Example of plasmid preparation of anti-CII chimeric antigen receptor encoding gene.
[0041] The plasmid preparation method of the anti-CII chimeric antigen receptor encoding gene of the present embodiment comprises the following steps:
[0042] (1) According to the order of Leader-scFv(CII)-CD8-2B4-CD3ζ-T2A-CTLA4-T2A-RQR8, entrust Sangon Bioengineering (Shanghai) Co., Ltd. to synthesize the entire expression cassette and insert the lentiviral plasmid pLent-C Between the AsiSI and NotI restriction sites of -GFP, the plasmid pLent-CII-CTLA4 was obtained. Finally, the correctly sequenced E.coli Top10 containing the lentiviral plasmid pLent-CII-CTLA4 was obtained. The lentiviral plasmid pLent-CII-CTLA4 was extracted using an endotoxin-free plasmid extraction and purification kit. After the concentration of the plasmid was determined, it was stored in a -20°C refrigerator for later use.
[0043] (2) Use the endotoxin-free plasmid extraction and purification kit (purchased...
Embodiment 3
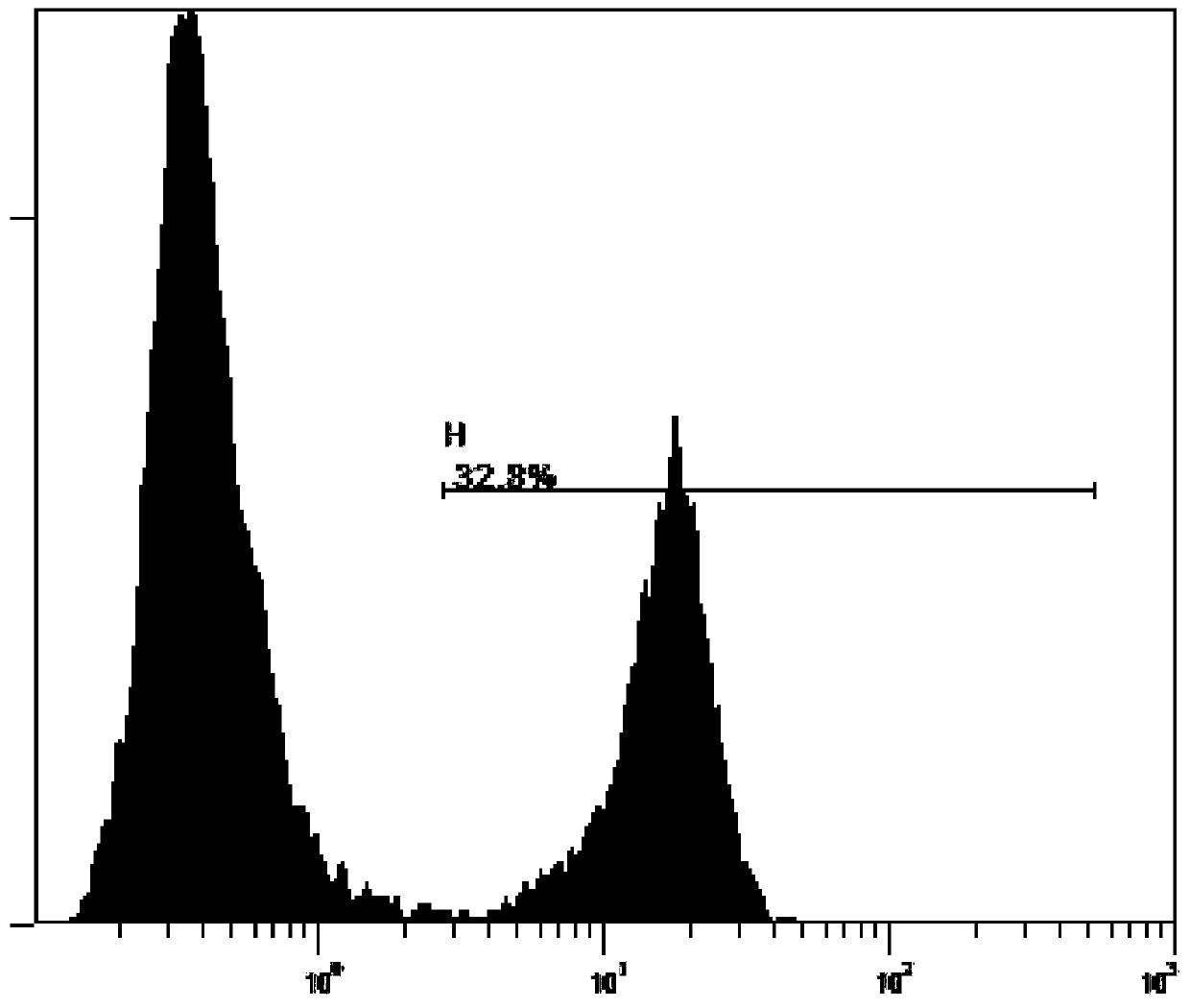
[0045] The present invention provides an example of Treg immune cells having anti-CII chimeric antigen receptor coding genes. The preparation method comprises: first packaging the pLent-CII-CTLA4 plasmid with lentivirus, and then using the recombinant lentivirus to infect Tregs immune cells.
[0046] (1) Lentiviral packaging, titer detection
[0047] The Lentiviral Packaging Kit lentiviral packaging kit was used, and the specific method was as follows: Inoculate the lentiviral packaging cell line 293T in a 10 cm culture dish containing DMEM+10% FBS, culture at 37°C, 5% CO2, and the adherence rate was 70%. %-80% ready for transfection. Take a sterile 1.5ml EP tube or 15ml centrifuge tube, and prepare the reaction system according to the following components: serum-free DMEM: 4ml; pLent-CII-CTLA4 plasmid: 10μg; GM easyTM Lentiviral Mix: 10μl (10μg); HG TransgeneTM Reagent: 60 μl. After mixing, place it at room temperature for 20 minutes, evenly drop it into the culture dish c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com