Iridium complex phosphorescent material based on main ligand containing rigid aromatic amine functional group
A technology of functional groups and iridium complexes, applied in the field of organic light-emitting materials, can solve the problems of low performance of organic light-emitting diodes and affect the application of organic light-emitting diodes, and achieve the effect of inhibiting non-radiative transitions and enhancing molecular rigidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
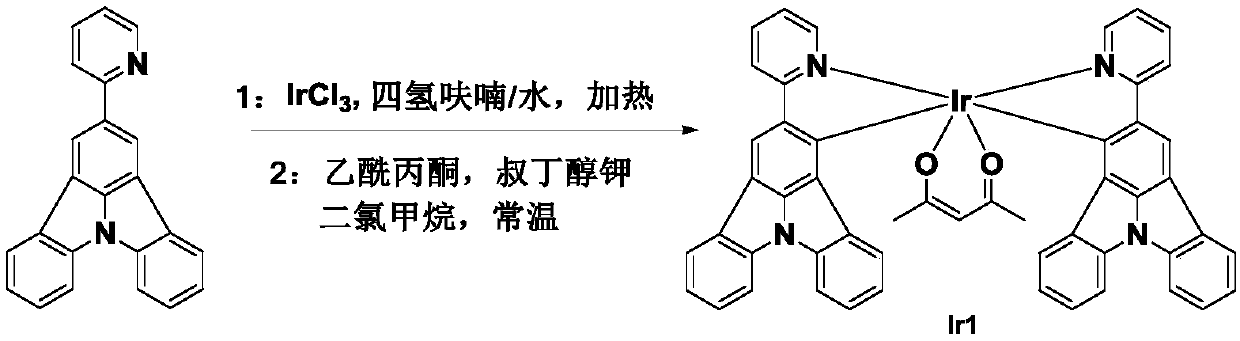
[0021] The organometallic iridium complex phosphorescent material Ir1 of the present embodiment has a chemical formula of C 51 h 33 IrN 4 o 2 , the molecular structure formula is:
[0022]
[0023] Refer to attached figure 1 , the synthesis steps are as follows:
[0024] The first step: 0.16g organic ligand Put 0.08g of iridium trichloride into the reaction vessel, add 30mL of tetrahydrofuran and water mixed solvent in nitrogen atmosphere, the volume ratio of tetrahydrofuran and water in the mixed solvent is 3:1, heat to 110°C in nitrogen atmosphere, and stir for 12 hours Cool to room temperature, separate the organic layer with a separatory funnel; dry the organic layer with anhydrous sodium sulfate, and remove the solvent by rotary evaporation to obtain an intermediate product;
[0025] The second step: under the condition of normal temperature nitrogen, dissolve 0.035g potassium tert-butoxide and 0.035g acetylacetone in 20mL dichloromethane, stir for 0.5h, then ad...
Embodiment 2
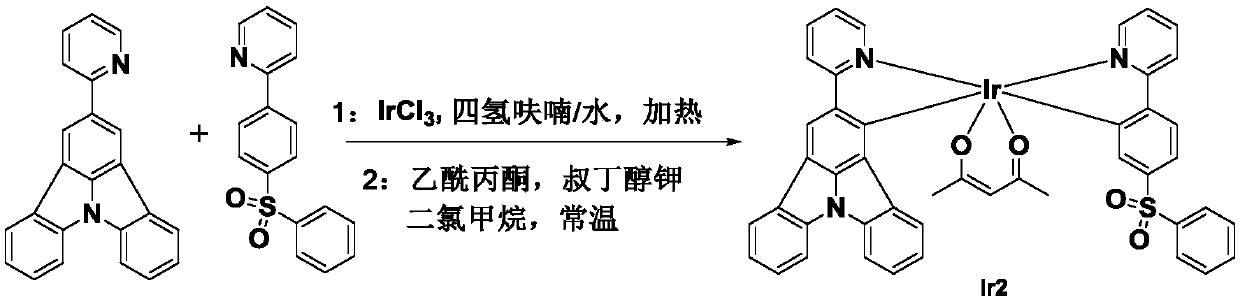
[0029] The organometallic iridium complex phosphorescent material Ir2 of the present embodiment has a chemical formula of C 45 h 32 IrN 3 o 4 S, the molecular structure formula is:
[0030]
[0031] Refer to attached figure 2 , the synthesis steps are as follows:
[0032] The first step: 0.083g organic ligand 0.077g organic ligand Put 0.08g of iridium trichloride into the reaction vessel, add 30mL of tetrahydrofuran and water mixed solvent in nitrogen atmosphere, the volume ratio of tetrahydrofuran and water in the mixed solvent is 3:1, heat to 110°C in nitrogen atmosphere, and stir for 12 hours Cool to room temperature, separate the organic layer with a separatory funnel; dry the organic layer with anhydrous sodium sulfate, and remove the solvent by rotary evaporation to obtain an intermediate product;
[0033] The second step: under the condition of normal temperature nitrogen, dissolve 0.035g potassium tert-butoxide and 0.035g acetylacetone in 20mL dichlorometh...
Embodiment 3
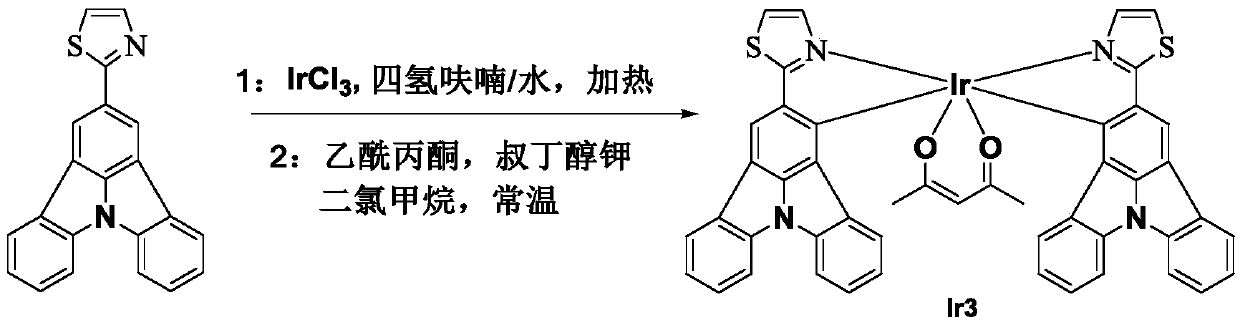
[0037] The organometallic iridium complex phosphorescent material Ir3 of this embodiment has a chemical formula of C47H29IrN4O2S2 and a molecular structural formula of:
[0038]
[0039] Refer to attached image 3 , the synthesis steps are as follows:
[0040] The first step: 0.2g organic ligand Put 0.098g of iridium trichloride into the reaction vessel, add 30mL of tetrahydrofuran and water mixed solvent in nitrogen atmosphere, the volume ratio of tetrahydrofuran and water in the mixed solvent is 3:1, heat to 110°C in nitrogen atmosphere, and stir for 12 hours Cool to room temperature, separate the organic layer with a separatory funnel; dry the organic layer with anhydrous sodium sulfate, and remove the solvent by rotary evaporation to obtain an intermediate product;
[0041] The second step: under the condition of normal temperature nitrogen, dissolve 0.108g potassium tert-butoxide and 0.115g acetylacetone in 20mL dichloromethane, stir for 0.5h and then add the interme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com