Fluorescent probe for detecting hydrogen sulfide based on drug molecules and preparation method thereof
A technology of fluorescent probes and drug molecules, applied in the field of chemical analysis and detection, can solve the problems of 3-hydroxythalidomide and other problems, and achieve high selectivity and anti-interference ability, strong selectivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
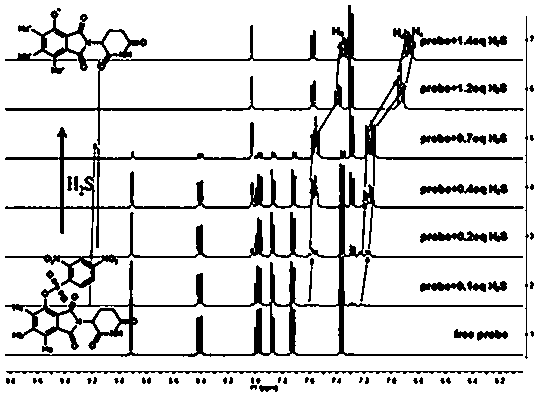
[0014] Example 1 Preparation of (2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl)2,4-dinitrobenzenesulfonate
[0015] 3-Hydroxythalidomide (137 mg, 0.5 mmol) and N,N-diisopropylethylamine (130 mg, 1.0 mmol) were dissolved in dichloromethane (10 mL), cooled to 0°C, and divided 2,4-Dinitrobenzenesulfonyl chloride (160 mg, 0.6 mmol) was added in batches, stirred at room temperature under argon atmosphere for 16 h, then concentrated, and the pure probe product was obtained by column chromatography (yield: 148 mg, Yield: 58.8%).
[0016] 1 H NMR (400 MHz, DMSO- d6 ), δ : 11.13(s, 1H), 8.95(d, J = 2.8 Hz, 1H), 8.44 (dd, J = 9.2, 2.8 Hz, 1H), 8.07-7.97 (m, 1H), 7.91 (d, J = 7.3 Hz, 1H),7.76 (d, J = 8.1 Hz, 1H), 7.40 (d, J = 9.2 Hz, 1H), 5.09 (dd, J = 12.9, 5.3Hz, 1H), 2.94-2.75 (m, 1H), 2.63-2.52 (m, 1H), 2.47-2.31 (m, 1H), 2.05-1.92(m, 2H).
[0017] 13 C NMR (100 MHz, DMSO- d6 ) δ : 173.10, 170.02, 166.63, 164.71, 154.27, 149.80, 142.72, 139.86, 138.32, 134.3...
Embodiment 2
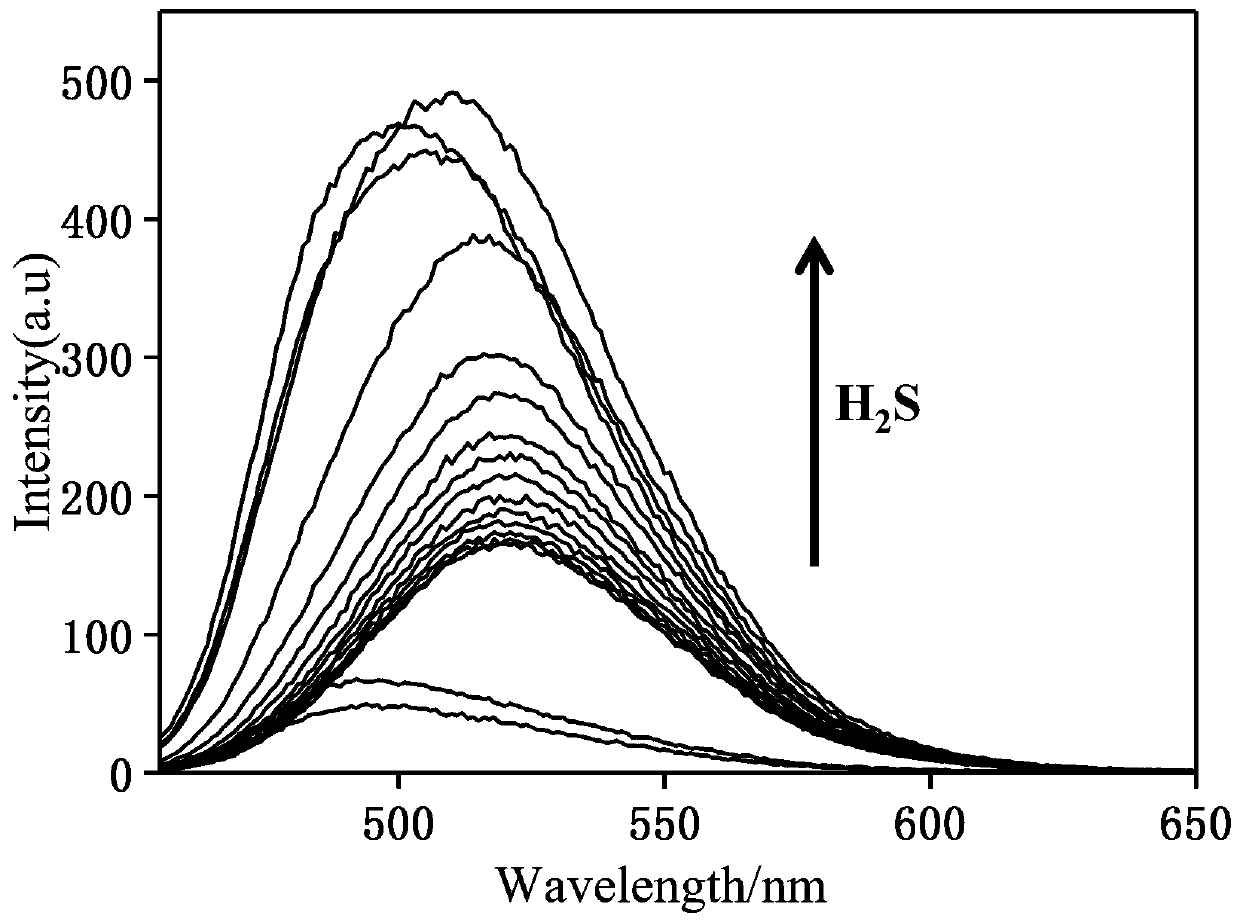
[0019] The change of fluorescence spectrum of the synthesized hydrogen sulfide fluorescent probe with the increase of NaHS addition equivalent
[0020] The hydrogen sulfide fluorescent probe prepared in Example 1 was dissolved in acetonitrile, diluted with PBS buffer solution, and NaHS standard solutions of different equivalents (0-30eq) were added to measure its fluorescence properties. Fluorescence spectrum such as figure 2 . It can be seen from the figure that the fluorescence intensity increases with the increase of NaHS addition equivalent.
Embodiment 3
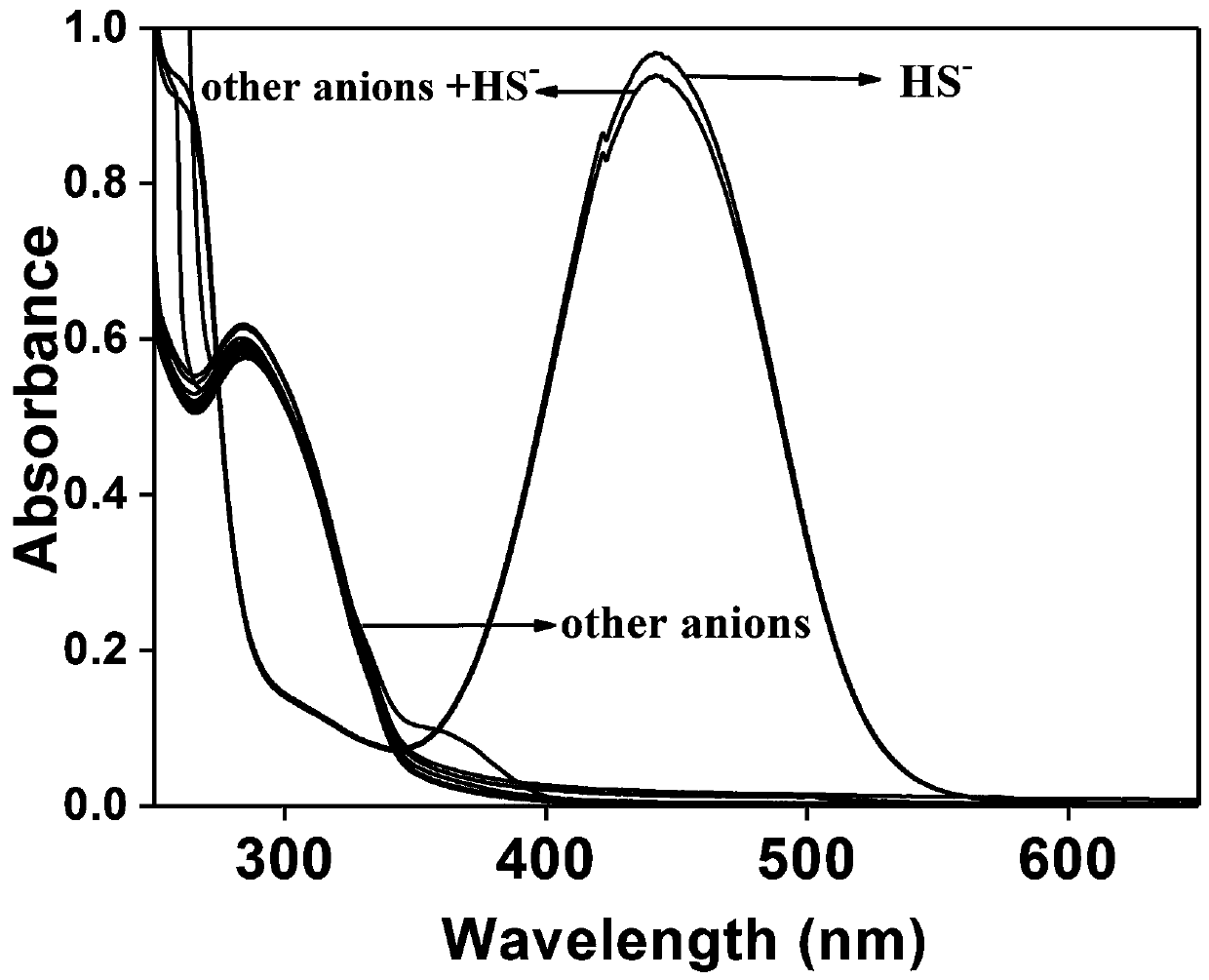
[0022] Selectivity of Synthetic Fluorescent Probes for Hydrogen Sulfide to Different Ions
[0023] In order to verify whether the probe has specific selectivity and strong anti-interference ability, we selected various anions (Cl - , AcO - , H 2 PO 4 - , HCO 3 - , CO 3 2- , H 2 PO 4 - , SO 4 2- , Br - , I - , NO 2- , NO 3- , HS - , F - ) and perform a fluorescence test. It was found that there was little change in the fluorescence intensity, but the fluorescence of the probe was significantly enhanced after adding NaHS. Therefore, the probe of the present invention can be used as a method for detecting H 2 S is a highly selective fluorescent probe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com