Benzoyl hydrazone neuraminidase inhibitor and preparation method and application thereof
A technology of neuraminidase and benzoyl hydrazone, which is applied in the preparation of hydrazone, hydrazide, antiviral agent, etc., can solve the problems of single route of administration, high price of Tamiflu, difficult to popularize, etc., and achieve excellent Effect of Neuraminidase Inhibitory Activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
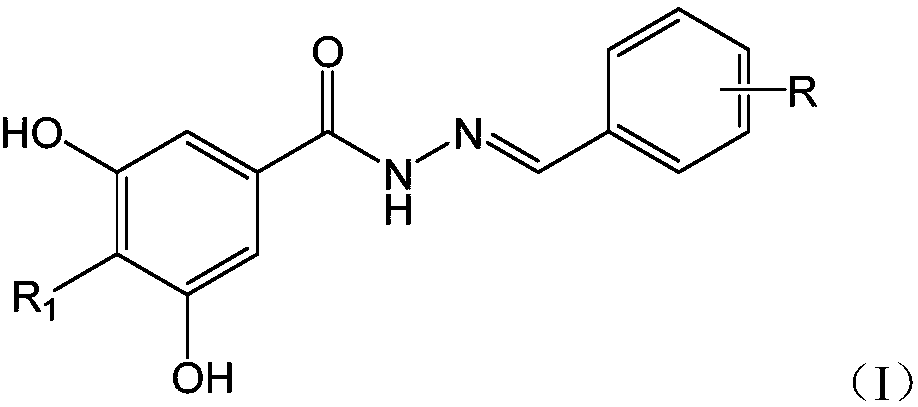
[0025] Embodiment 1, taking the synthetic compound R-1 as an example, its structural formula is as follows:
[0026]
[0027] (1) Accurately weigh 178.2mg (1.060mmol) of methyl 3,5-dihydroxybenzoate (compound shown in formula (II)) and 0.5mg (15.625mmol) of hydrazine hydrate into a round bottom flask, then add 5.0 mL of absolute ethanol was dissolved and mixed, stirred evenly, reacted at room temperature for 16 hours, concentrated, filtered, and dried to obtain the intermediate 3,5-dihydroxybenzoic acid hydrazide.
[0028] (2) Accurately weigh 180.0 mg (0.978 mmol) of 5-chloro-2-nitrobenzaldehyde (compound shown in formula (IV)), dissolve it in 5 ml of ethanol, and accurately weigh the intermediate 3,5-dihydroxybenzoic acid Add 164.0mg (0.978mmol) of hydrazide into the ethanol solution, then dropwise add 1-2 drops (0.05-0.1mL in total) of 99% acetic acid solution, stir well, react at room temperature for 6 hours, TLC (ethyl acetate : Petroleum ether=4:1) to monitor the com...
Embodiment 2-10
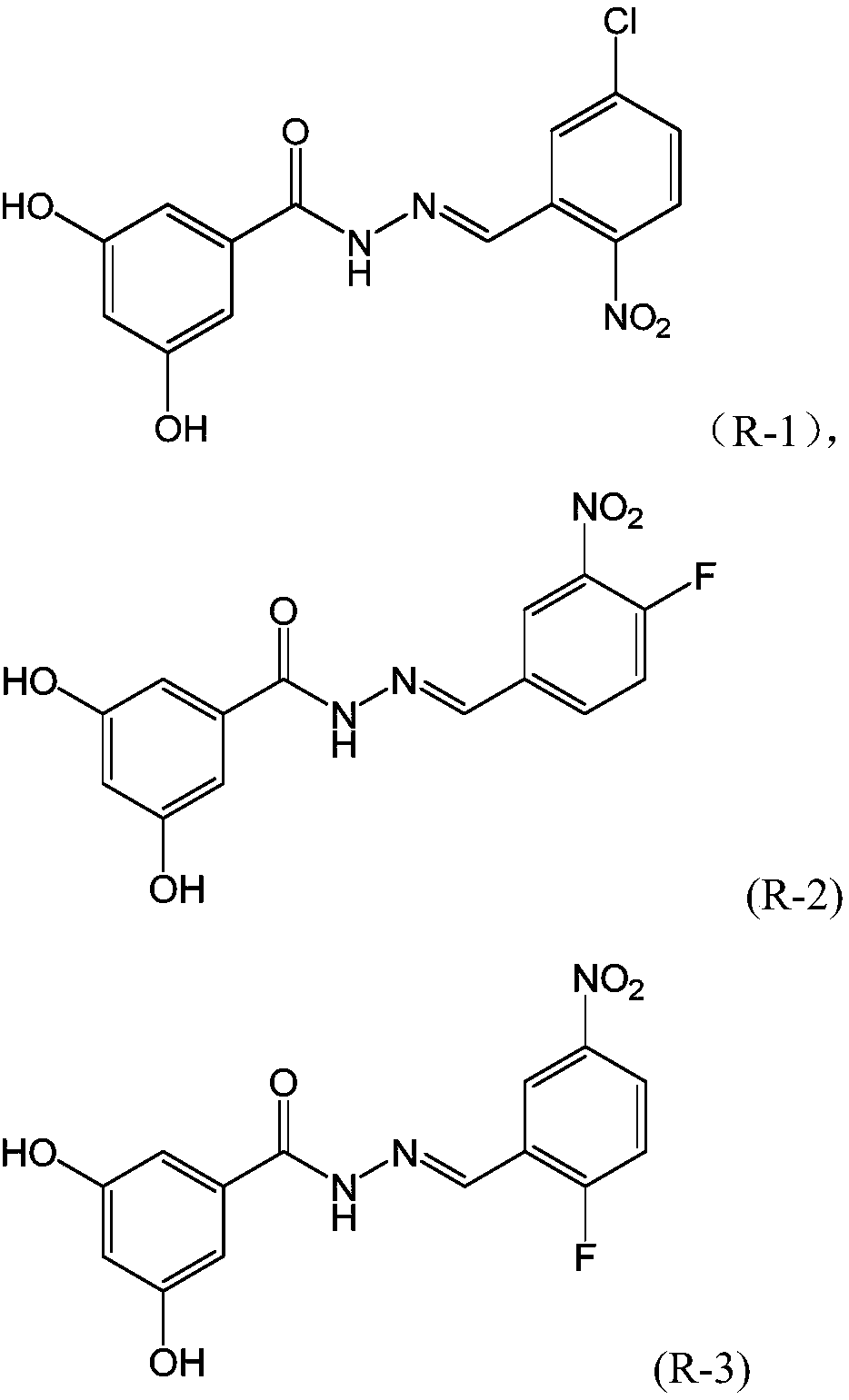
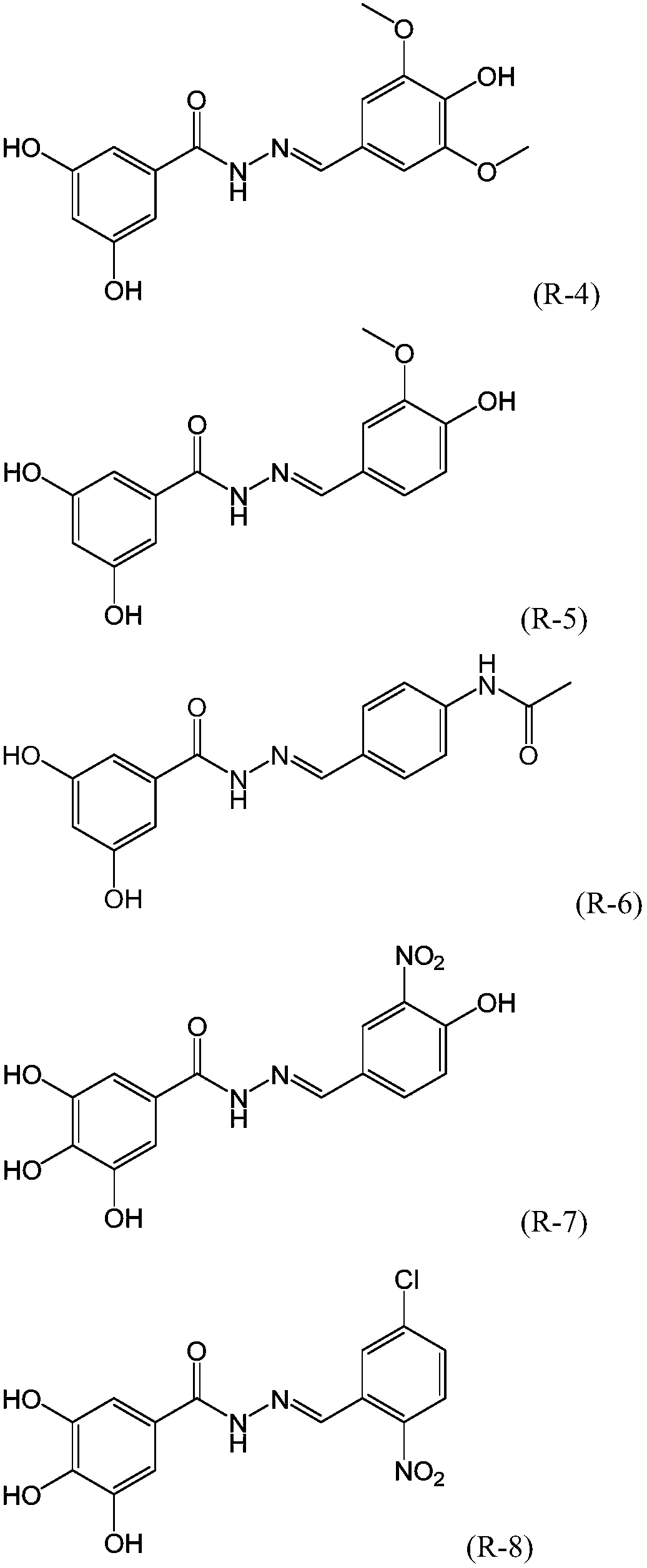
[0032] Compounds R2-10 were synthesized by a method similar to Example 1, wherein the compounds represented by formula (II) and formula (IV) were shown in the following table respectively, and the moles of each raw material were the same as those in Example 1.
[0033]
[0034]
[0035] R-2, yellow powder: 1 H NMR (500MHz, DMSO-d 6 ):δ11.95(s,1H),δ9.62(s,2H),8.49(s,1H),δ8.45(d,J=6.5Hz,1H),δ8.13(s,1H), δ7.70-7.65(m,1H); δ6.77(s,2H), δ6.45(s,1H),. 13 C NMR (125MHz, DMSO-d 6 ): δ164.02, 158.91, 156.61, 154.51, 144.62, 137.76, 135.59, 134.71, 132.38, 124.43, 119.56, 106.31. HRMS (ESI) calcd for C 14 h 10 FN 3 o 5 [M+H] + :320.067725; Found: 320.067734.
[0036] R-3, yellow powder: 1 H NMR (500MHz, DMSO-d 6 ):δ12.04(s,1H),δ9.53(s,2H),8.57(s,2H),δ8.32-8.33(m,1H),δ7.61(t,J=7.5Hz,1H ); δ6.76(s,2H), δ6.45(s,1H),. 13 C NMR (125MHz, DMSO-d 6 ): δ165.06, 163.71, 162.73, 158.77, 144.54, 138.28, 135.01, 127.11, 123.76, 121.53, 117.87, 106.30. HRMS (ESI) calcd for C 14 h ...
Embodiment 11
[0044] Embodiment 11, experiment of inhibiting neuraminidase activity
[0045] 1. Experimental instruments and materials
[0046] Multifunctional fluorescent microplate reader, SP-Max 3500FL, Shanghai Shanpu Biotechnology Co., Ltd.;
[0047] Ultra-clean workbench;
[0048] Bond A3Pipette manual single-channel adjustable pipette, 0.5-10μL, 10-100μL, Titan Technology;
[0049] 96-well plate (black), sterilized, Corning;
[0050] Neuraminidase Inhibitor Screening Kit, P0309, Biyuntian Biotechnology, including: Neuraminidase Detection Buffer, 10ml; Neuraminidase, 1ml; Neuraminidase Fluorescent Substrate, 1ml; Milli-Q Water , 1.2ml;
[0051] Positive control drug: oseltamivir acid, Shanghai Hekang Biotechnology Co., Ltd.
[0052] 2. Experimental method
[0053] Prepare the initial concentration of the positive control drug and the target compound to be 1000 μm / l, and dilute it into 7 concentration gradients according to the doubling ratio, which are 1000 μm / l, 200 μm / l, 40 μm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com