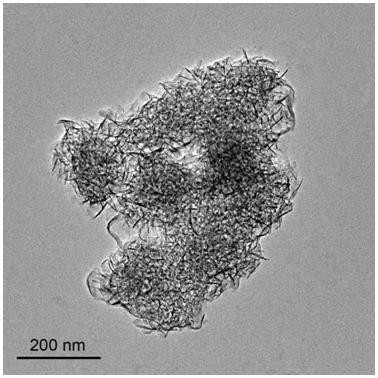
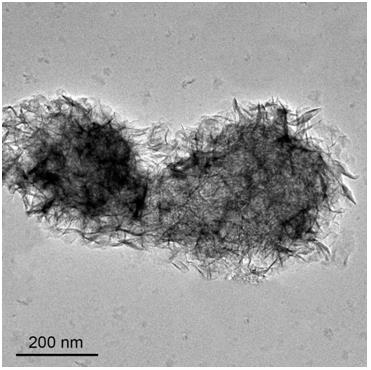
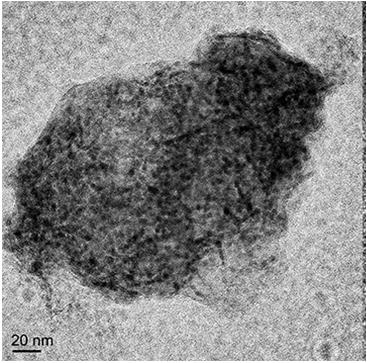
A method for in-situ preparation of highly dispersed metal catalysts by growing two-dimensional nanosheets
A two-dimensional nanotechnology for preparing metals, which is applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc. issues such as being effectively protected, achieving the effects of low cost, small scale, and efficient and easy methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Dissolve 3.6349g nickel nitrate hexahydrate in 10ml deionized water to prepare salt solution A1, impregnate A1 solution in 7.3363g commercial γ-Al 2 o 3 and ultrasonically disperse for 30 minutes, dry at 120°C for 2 hours, pass through nitrogen protection, place in an ice-water bath, and mechanically stir vigorously; prepare a reducing agent mixture B1 containing 1.8915 g of sodium borohydride, 0.2 g of sodium hydroxide and 25 ml of water . Add the prepared reducing agent mixture B1 dropwise to the above impregnation system with a peristaltic pump. The dropwise addition time is 90 minutes. After the dropwise addition, continue to react for 2 hours to wait for sufficient reduction. Suction filter the reacted product, and then filter it with deionized water and dehydrated ethanol until the filtrate is neutral, then put it into a beaker with 200ml dehydrated ethanol and stir it openly, the stirring speed is 400r / min, and the stirring time 16h, the catalyst precursor of n...
Embodiment 2
[0026] Dissolve 5.0500g of ferric nitrate nonahydrate in 8ml of deionized water to prepare salt solution A, impregnate solution A in 6.1136g of commercial SiO 2 and ultrasonically disperse for 30 minutes, dry at 120°C for 2 hours, pass through nitrogen protection, place in an ice-water bath, and mechanically stir vigorously; prepare a reducing agent mixture B containing 1.8915 g of sodium borohydride, 0.2 g of sodium hydroxide and 25 ml of water . Add the prepared reducing agent mixture B to the above impregnation system dropwise with a peristaltic pump. The dropwise addition time is 90 minutes. After the dropwise addition, continue to react for 2 hours to wait for sufficient reduction. Suction filter the reacted product, and then filter it with deionized water and dehydrated ethanol until the filtrate is neutral, then put it into a beaker with 200ml dehydrated ethanol and stir it openly, the stirring speed is 400r / min, and the stirring time For 16h, the catalyst precursor of...
Embodiment 3
[0028] Dissolve 3.6379g of cobalt nitrate hexahydrate in 28ml of deionized water to prepare salt solution A, impregnate solution A in 4.8911g of commercial TiO 2and ultrasonically disperse for 30 minutes, dry at 120°C for 2 hours, pass through nitrogen protection, place in an ice-water bath, and mechanically stir vigorously; prepare a reducing agent mixture B containing 1.8915 g of sodium borohydride, 0.2 g of sodium hydroxide and 25 ml of water . Add the prepared reducing agent mixture B to the above impregnation system dropwise with a peristaltic pump. The dropwise addition time is 90 minutes. After the dropwise addition, continue to react for 2 hours to wait for sufficient reduction. Suction filter the reacted product, and then filter it with deionized water and dehydrated ethanol until the filtrate is neutral, then put it into a beaker with 200ml dehydrated ethanol and stir it openly, the stirring speed is 400r / min, and the stirring time For 16h, the catalyst precursor of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com