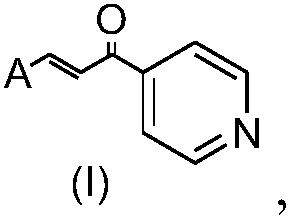
Application of heterocyclic acrylketone type compound as antibacterial agent
A technology of acrylone and compounds, applied in the field of medicine, can solve problems such as ineffective colistin-resistant bacteria and unknown systemic infection models
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: the mensuration of compound antibacterial spectrum
[0070] According to the method recommended by CLSI, the minimum inhibitory concentration (MIC) was determined by plate double dilution method and multi-point inoculator. Compounds PFK-158, PFK-015 and 3PO of the present invention were all purchased from Shanghai Taosu Biochemical Technology Co., Ltd.; reference substances (levofloxacin, polymyxin E, polymyxin B, etc.) were all purchased from China Food and Drug Administration Research Institute of Standardization and Standardization Institute. Drugs etc. are all diluted into various required concentrations with broth twice, add appropriate amount to the plate respectively, after the agar medium is melted, pour quantitatively into the plate containing the drug solution and mix evenly, the compound of the present invention (such as the compound prepared in the examples) The final concentrations of the control substance and the reference substance were 0.0...
Embodiment 2
[0077] Example 2: Determination of compound polymyxin E combined with MIC by checkerboard method
[0078] Strains frozen at -70°C were streaked on nutrient agar plates and cultured overnight at 37°C. Pick 3 to 5 well-separated and uniformly shaped colonies from the agar petri dish and inoculate them into a small glass test tube containing 3 mL of CAMHB, and incubate at 37°C and 200 rpm for 6 to 7 hours. Take the bacterial liquid in the exponential growth phase and measure the McFarland concentration of the bacterial liquid in each test tube with a McFarland turbidimeter, and then use 0.85% NaCl solution to adjust the bacterial liquid to 0.5 McFarland, about (1~2)×10 8 CFU / mL. The bacterial solution was used within 15 minutes after preparation. Take the prepared polymyxin E and dilute it to an appropriate concentration with CAMHB for later use. Add 100 μL of CAMHB to the 96-well plate, add the same volume of the above antibiotics to the first column, and serially dilute them...
Embodiment 3
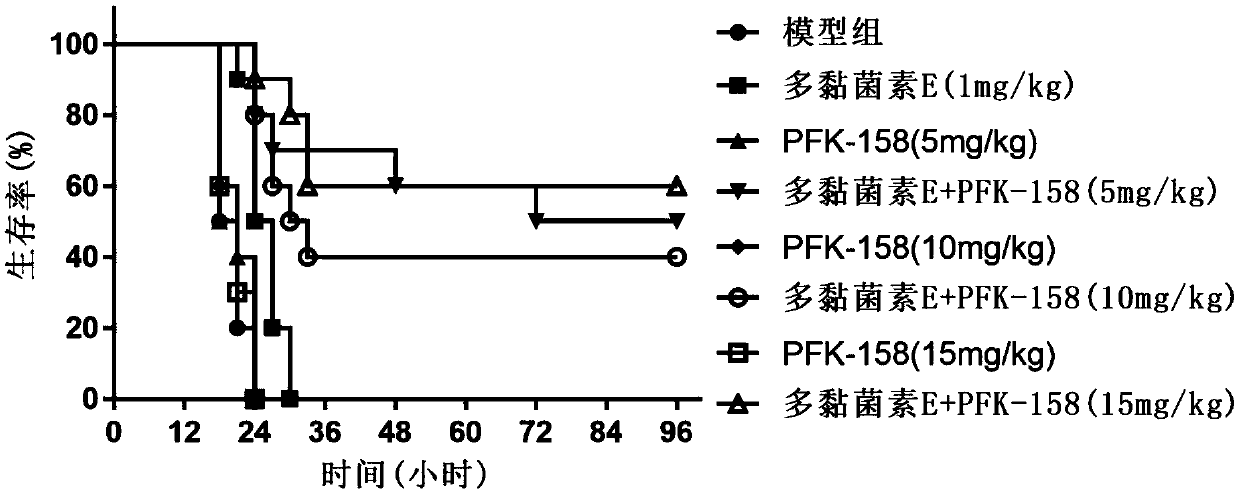
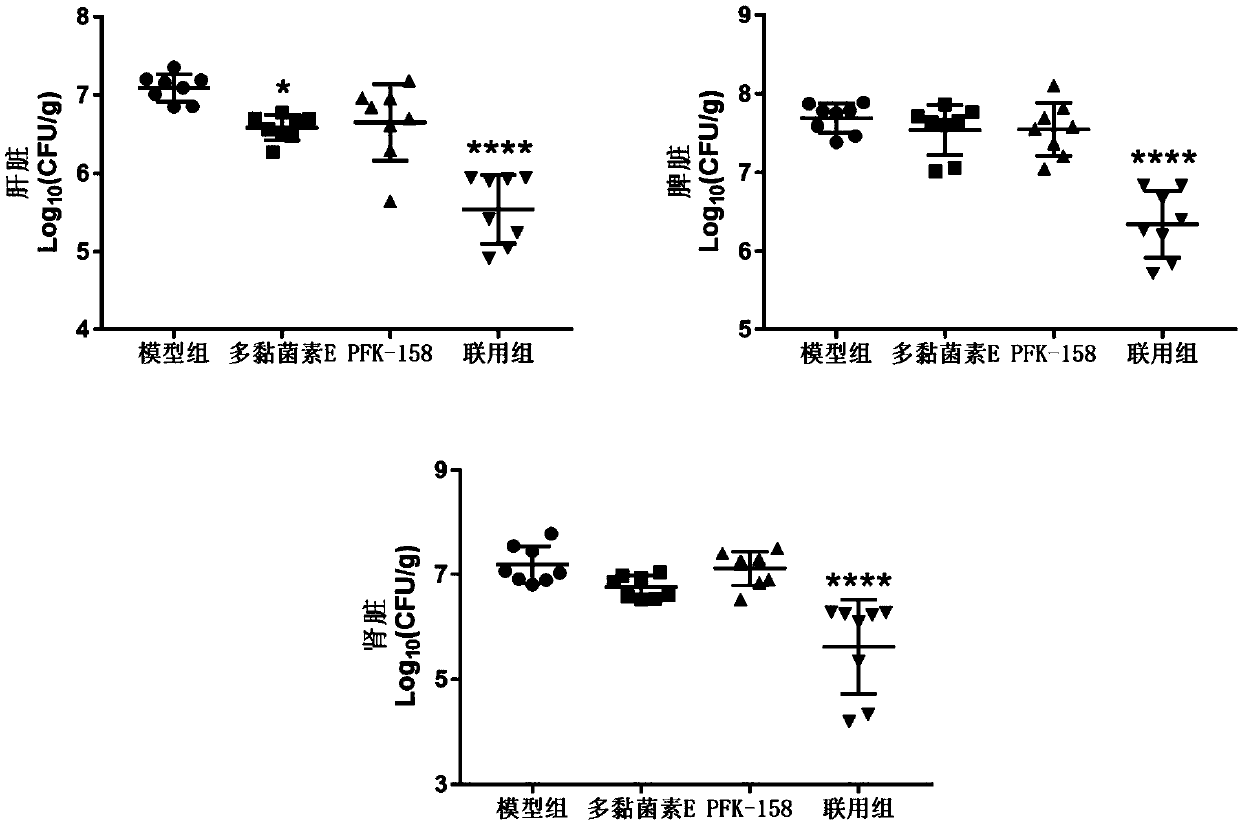
[0082] Example 3: PFK-158 combined with polymyxin E against systemic infection in mice caused by E.cloacae D01
[0083] Inoculate an appropriate amount of frozen-preserved bacteria from E.cloacae D01, a high-level polymyxin-resistant strain, into 10 mL of Zeng Bacteria Decoction, and culture it at 37°C for 6 hours. Cultivate statically at ℃ for 18 hours; the obtained bacterial liquid is properly diluted with 5% dry active yeast solution to prepare the infectious bacterial liquid for use; at the same time, it is diluted several times with 0.85% NaCl, and 10 μL is dripped on the MH plate, and left to dry Colony counting after inversion culture; female mice were randomly divided into groups according to body weight, 10 in each group; the abdomen was disinfected with iodine, and the mice were lightly inverted and intraperitoneally injected with 0.5mL of bacterial solution; 1mg / kg was prepared with 0.85% NaCl solution Polymyxin E alone and combined with 5, 10, and 15 mg / kg PFK-158 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com