Application of indoleacrylic acid in preparing drug for preventing and treating rheumatoid arthritis
A technology of indoleacrylic acid and rheumatoid, which is applied in the direction of drug combination, antipyretics, anti-inflammatory agents, etc., and can solve problems such as pulmonary interstitial lesions, difficult to cure, and incurable diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
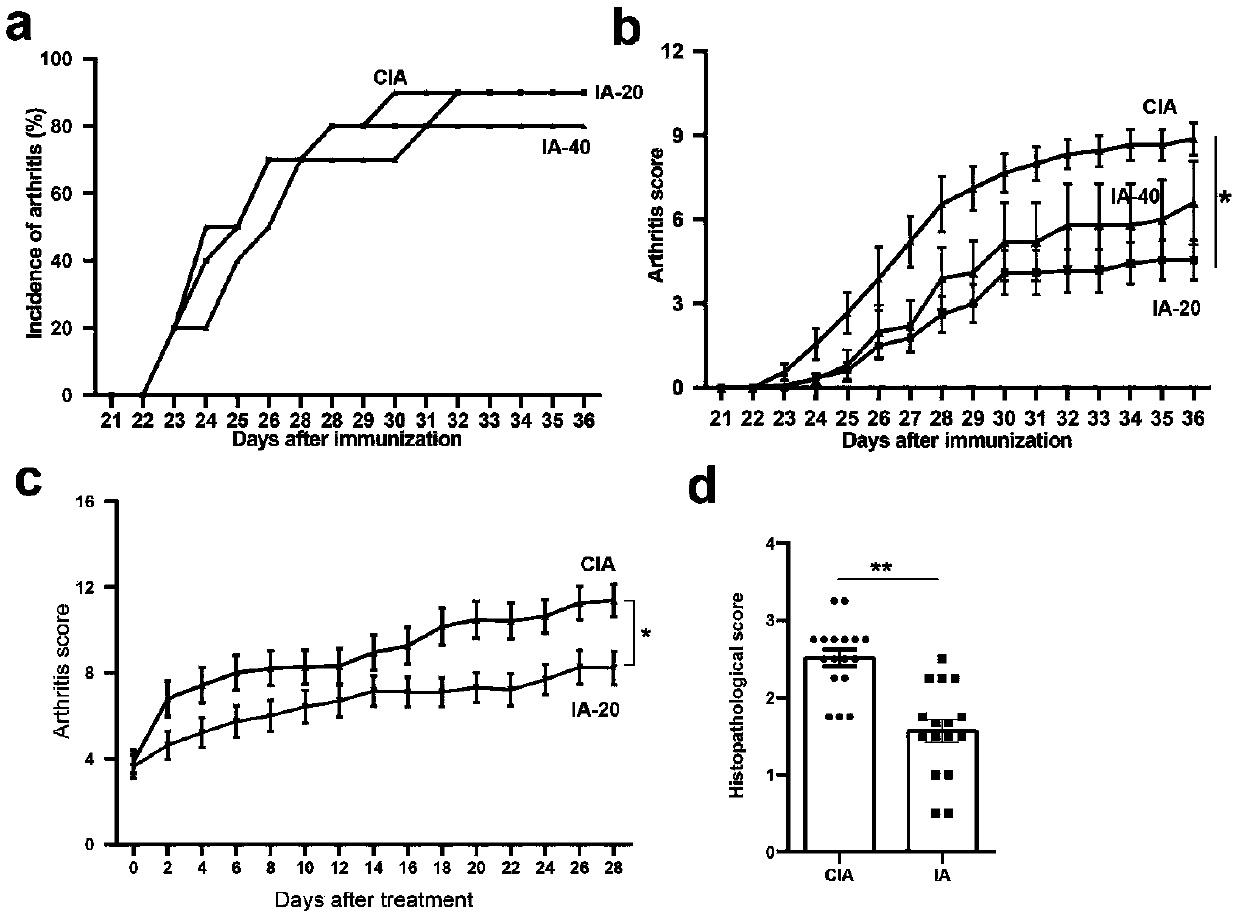
[0029] Indoleacrylic acid significantly inhibits the development of experimental arthritis
Embodiment approach
[0031] (1) Constructing a collagen-induced joint (CIA) model: 1) Dissolving collagen, dissolving bovine type II collagen (CII) in 0.1mol glacial acetic acid, adjusting the final concentration of CII to 4mg / mL, and overnight at 4°C. 2) Emulsifying collagen, mixing completely dissolved CII with an equal volume of Freund's complete adjuvant (CFA) to fully emulsify the two to prepare a collagen emulsion with a final concentration of 2 mg / mL. 3) For the first immunization, the base of the tail of the mice was depilated, and a total of 200 μg of collagen emulsion was injected intradermally at multiple points. 4) Booster immunization. On the 21st day after the initial immunization, dissolve type II collagen in 0.1mol glacial acetic acid (concentration 4mg / mL), add an equal volume of incomplete Freund's adjuvant (IFA) to make collagen emulsion (final concentration 2mg / mL). A total of 100 μg of collagen emulsion was injected intradermally at multiple points at the base of the tail. ...
Embodiment 2
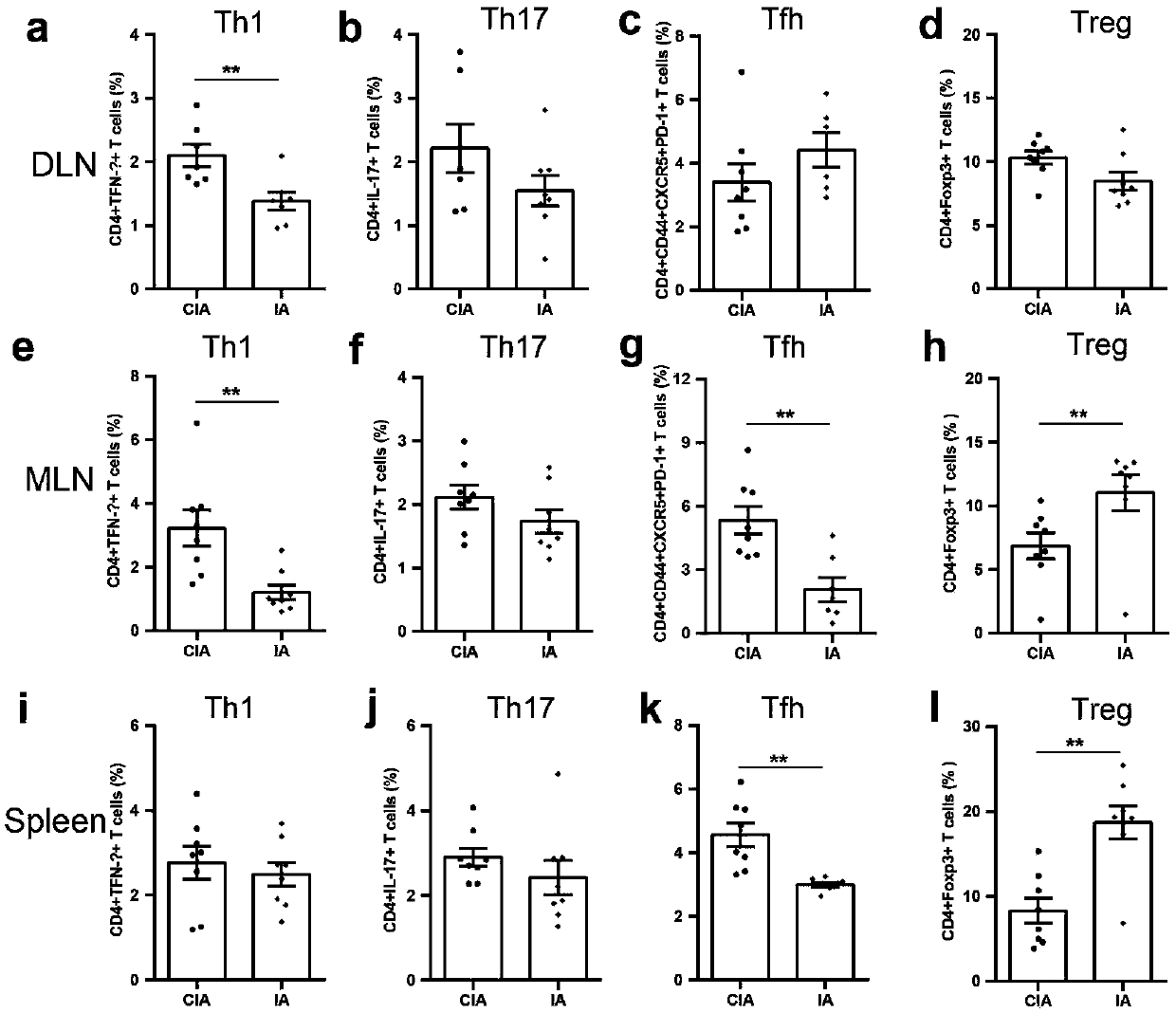
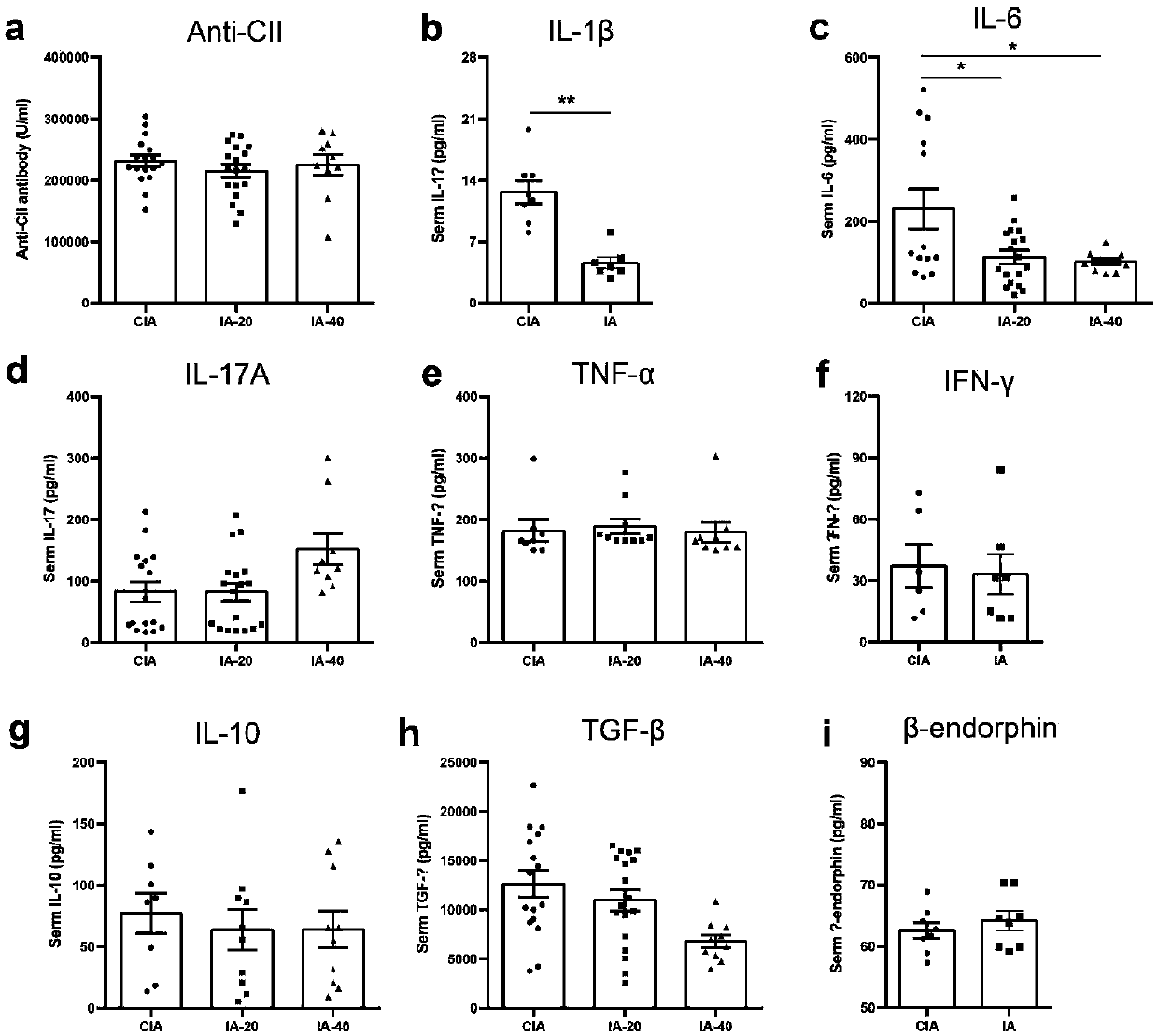
[0039] Indoleacrylic acid regulates the ratio of lymphocyte subsets in CIA mice
[0040] method of execution
[0041] Flow cytometry to detect the proportion of lymphocyte subsets: Isolate the joint draining lymph nodes (axillary and popliteal lymph nodes), mesenteric lymph nodes and spleen of CIA mice, use 1640 medium and filter to grind the lymph nodes into a single cell suspension, and adjust the number of cells 2-3 x 10 6 / 100μl, add staining flow antibody (eBioscience, U.S.A.), wash and resuspend, and use the machine (BDFACSAria TM II) Detection. see results figure 2 .
[0042] Research result
[0043] From figure 2 It can be concluded that the analysis of lymphocyte subsets showed that indoleacrylic acid significantly down-regulated the proportion of T helper cells Th1 in joint-draining lymph nodes (DLN) (attached figure 2 a, CIA group Th12.1±0.1755vs IA group Th11.383±0.1425), while the ratio of Th17 cells, follicular helper T cells Tfh and regulatory T cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com