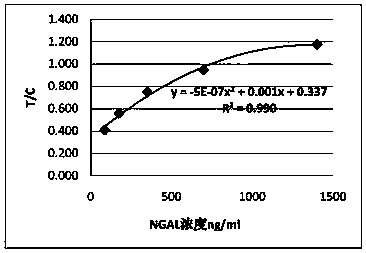
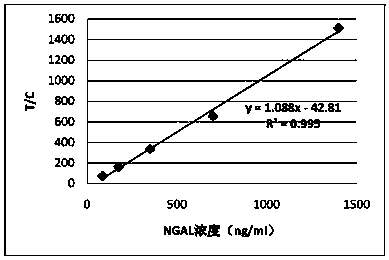
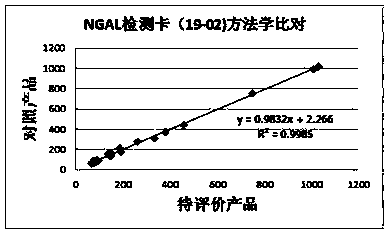
Anti-human neutrophil gelatinase-associated lipocalin (NGAL) antibodies and application thereof in test paper card
An antibody and neutrophil technology, applied in anti-animal/human immunoglobulin, application, biological testing and other directions, can solve the problems of narrow detection range, long detection period, small linear range of measurement, etc. The effect of convenient mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Preparation of anti-human neutrophil gelatinase-associated lipocalin hybridoma cell line
[0041] 1. Animal immunization
[0042]BALB / c female mice (purchased from Changzhou Cavens Experimental Animal Co., Ltd.) were immunized with recombinant human neutrophil gelatinase-associated lipocalin (recombinantly expressed in Escherichia coli, produced by our company) according to the general immunization procedure. For specific immunization conditions, please refer to the "Experimental Guidelines for Antibody Preparation and Use". The serum titer of immunized mice was tracked by indirect ELISA method, and the immunized mouse with the highest serum titer was selected for fusion experiment of mouse splenocytes and mouse myeloma cells.
[0043] 2. Cell Fusion
[0044] (1). Preparation of spleen cells
[0045] Take the immunized mice, remove their eyeballs, take blood, put them to death by breaking the cervical spine, soak them in 75% (v / v) alcohol for 10 minutes, t...
Embodiment 2
[0055] Example 2. Determination of the variable region sequence of the hybridoma cell line antibody
[0056] The variable region sequences of the above-mentioned hybridoma cell lines NG02 and NG19 were determined.
[0057] a. Extraction of RNA: Extract the total RNA of the above-mentioned hybridoma cell lines NG02 and NG19 with reference to the instructions of the Total Cell RNA Extraction Kit (purchased from Roche Company) and perform reverse transcription immediately;
[0058] b. Reverse transcription of RNA into DNA: Refer to Thermo Scientific Reverted First strand cDNASynthesis Kit (purchased from Thermo Company) to reverse-transcribe the total RNA extracted in the previous step to obtain cDNA, and freeze it at -20°C for later use;
[0059] c. PCR amplification and recovery of the variable region sequence: the cDNA obtained in the above step is used as a template, and the variable region sequence of the heavy chain and light chain is sequenced with the general primer for t...
Embodiment 3
[0063] Example 3. Recombinant expression and purification of single-chain antibody
[0064] According to the sequencing results in Example 2, a connecting peptide (GGGGS) 3 was added between the heavy chain and light chain variable regions of hybridoma cell lines NG02 and NG19, six histidines were introduced, and the entire gene was directly fused And the expression system of Pichia pastoris was used for the recombinant expression of the single chain antibody. The expressed antibodies were named as antibodies NG02 and NG19, respectively. The recombinant expression of the above-mentioned single-chain antibody is specifically as follows:
[0065] 1. Construction of single-chain antibody gene expression vector
[0066] The gene sequence of the single-chain antibody NG02 is shown in SEQ ID NO:19, and the amino acid sequence is shown in SEQ ID NO:17; the gene sequence of the single-chain antibody NG19 is shown in SEQ ID NO:20, and the amino acid sequence is shown in SEQ ID NO: 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com