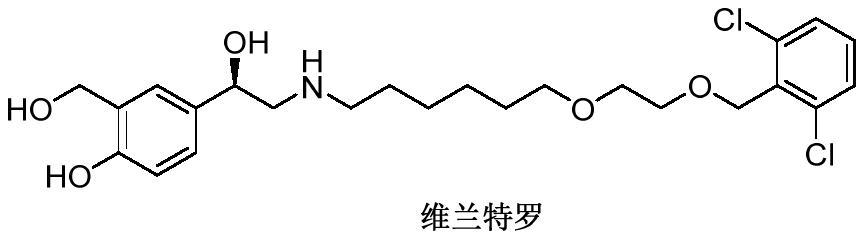
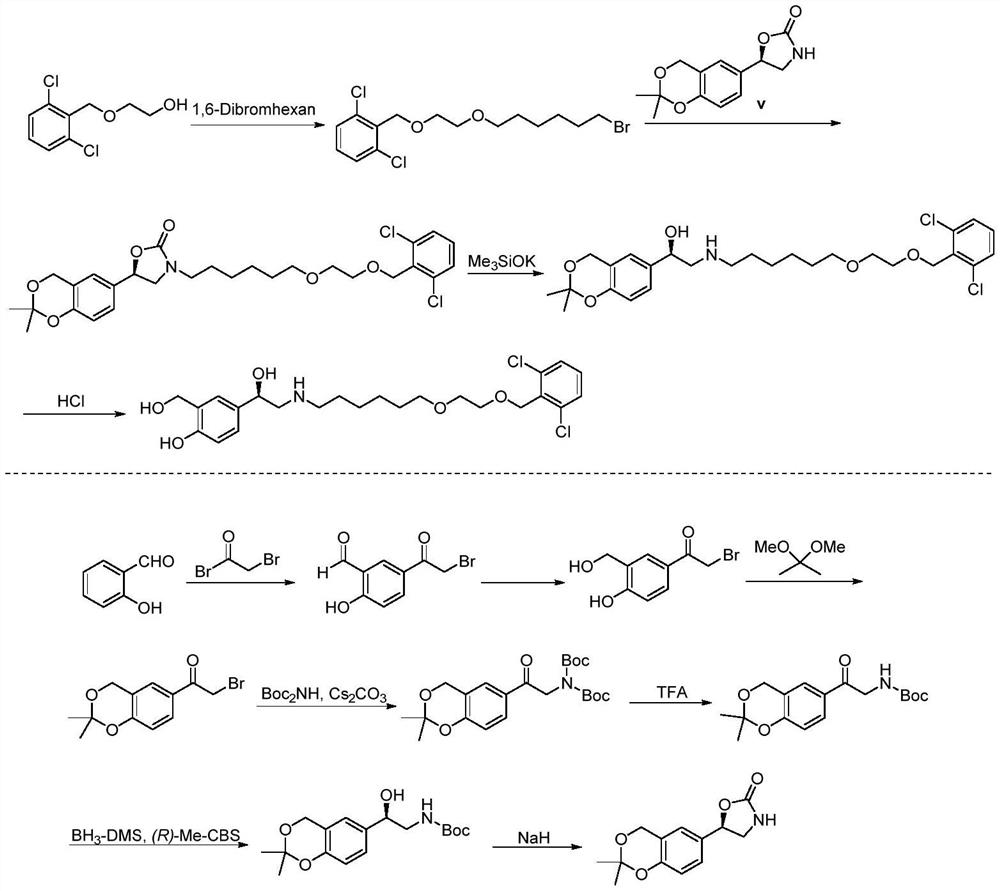
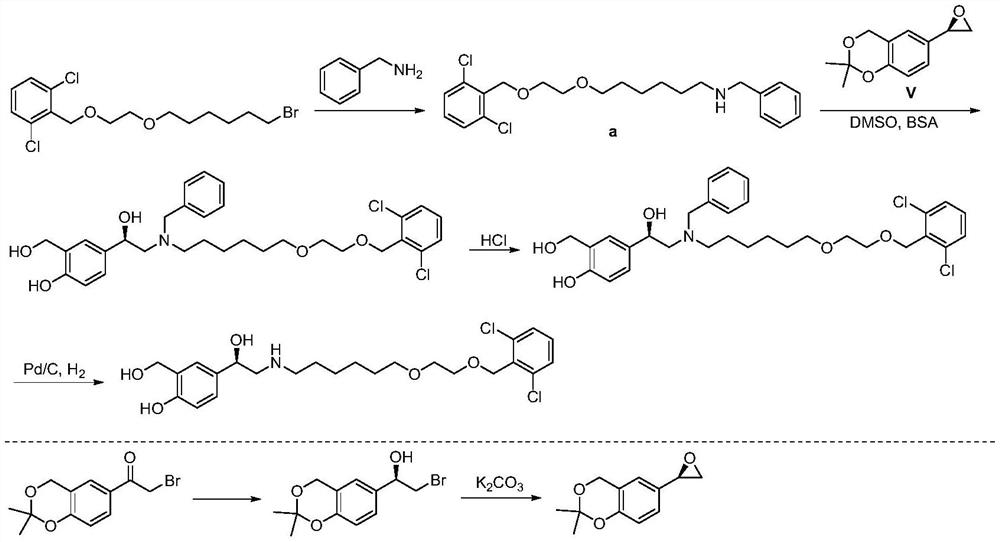
A kind of method for preparing vilanterol
A tetrahydrofuran, molar ratio technology, applied in the preparation of carboxylate, carboxylic acid amide, organic chemical methods, etc., can solve the problems of the difficulty of chiral purity to meet the quality requirements, the difficulty of chiral center construction, and the limitation of industrial applications. Achieve the effects of avoiding the use of protective groups, increasing atom utilization, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of Intermediate 1:
[0024] Add 200mL water to a 500mL three-necked flask, add 13.2g (1.1eq) sodium hydroxide in batches, cool to 0-5°C, add 37.2g (1eq) salicyl alcohol while stirring, and then add 0.68g (1%) Benzyltriethylammonium chloride, fully stirred for 1 hour, under the condition of 0-5 ℃, slowly added 44.4g of 50% glyoxylic acid aqueous solution into the reaction system dropwise within 1 hour, and the temperature of the reaction system rose to 25 ℃, continue to react for 6 hours, and the remaining raw materials in the liquid phase are less than 1%, and the reaction is completed. Cool the system to 0-5°C, adjust the pH to 2-3 with concentrated hydrochloric acid, extract with 100×2 mL of tert-butyl acetate, combine the organic phases, wash with 100 mL of saturated saline, 100 mL of water, dry over anhydrous magnesium sulfate, and filter. Concentration gave a white solid, and the crude product was recrystallized from toluene to obtain 50 g, with a yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com